Consolidated Guidelines
2.4 Prevention of TB
2.4.1 TB preventive treatment
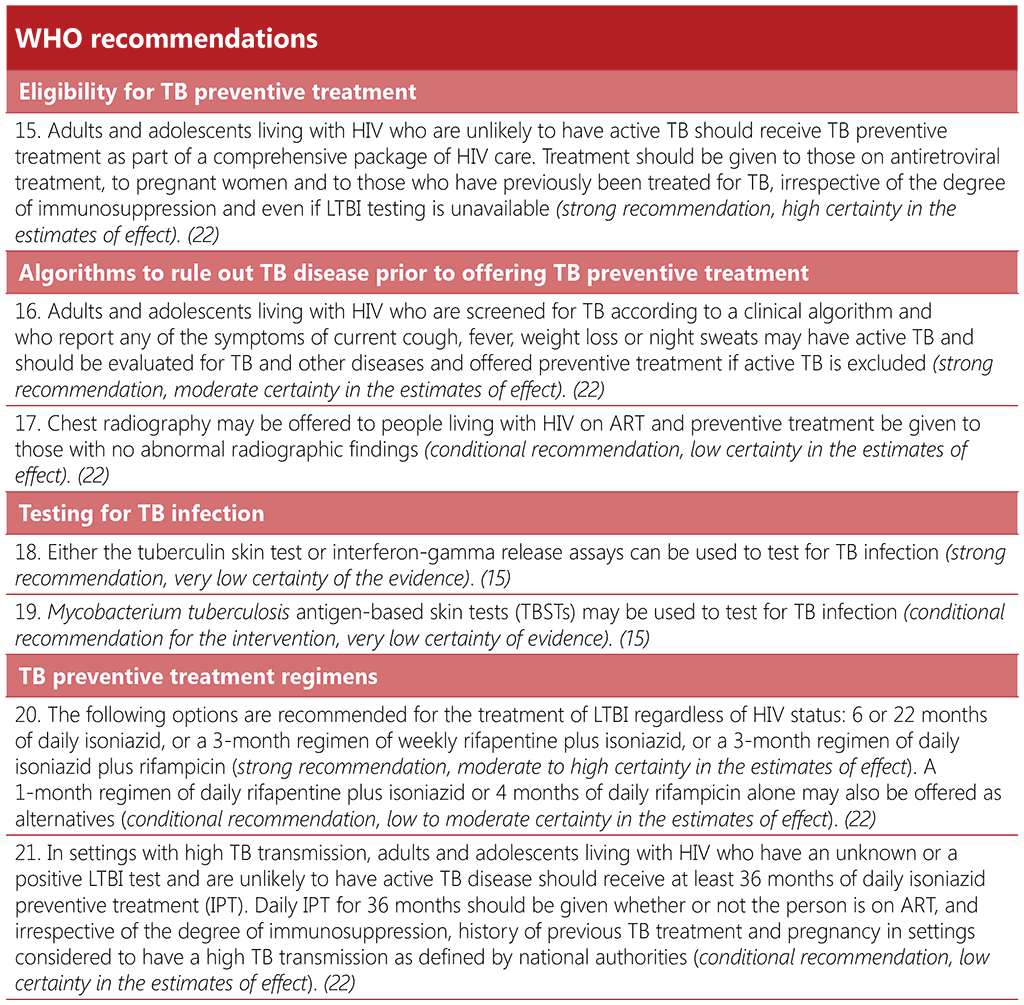
2.4.1.1 Background
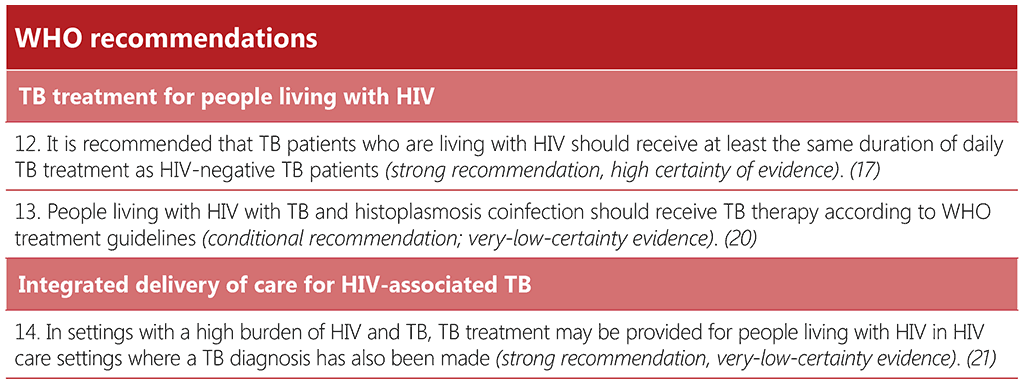
2.2 TB diagnosis
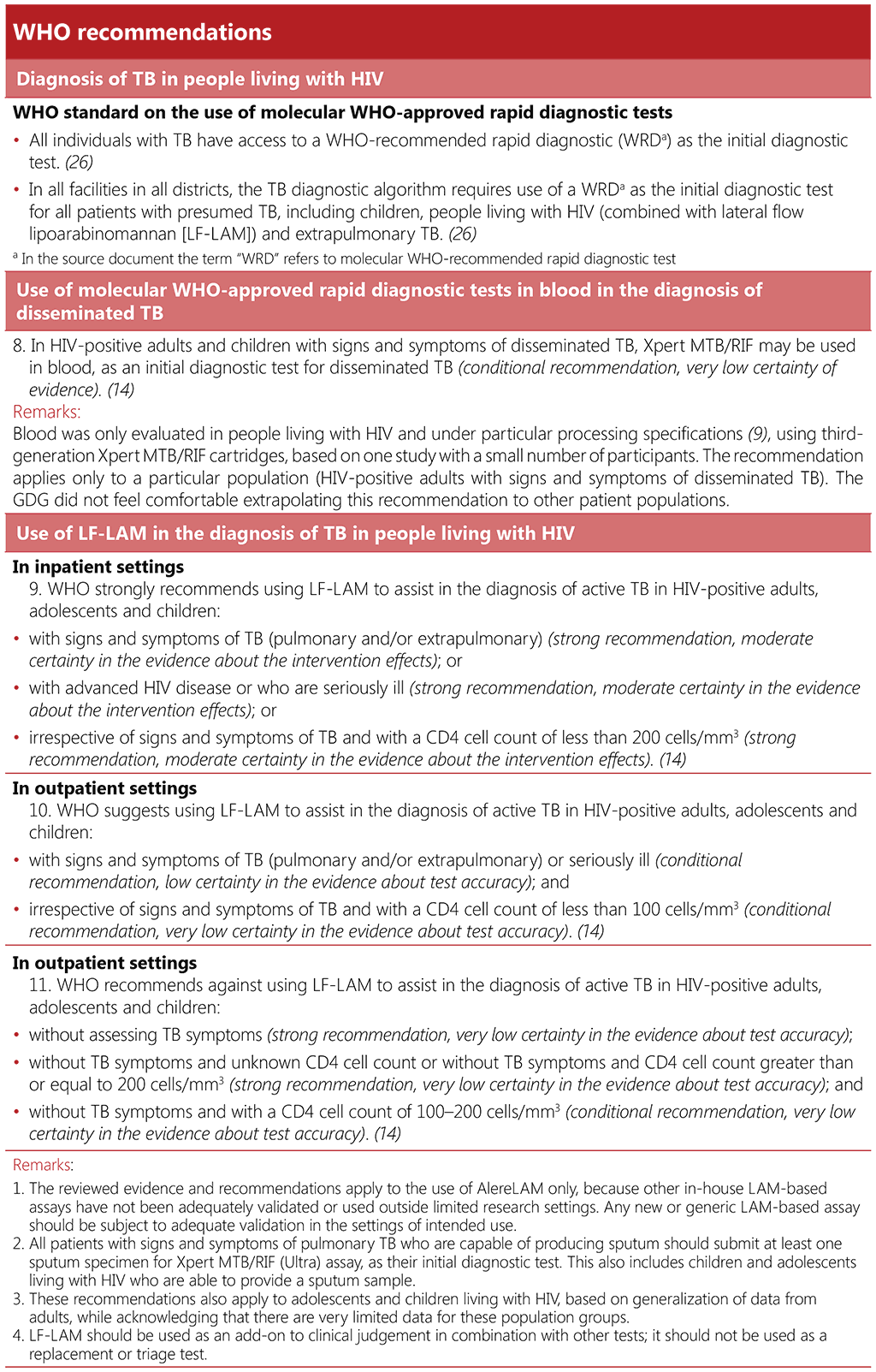
2.2.1 Background
People living with HIV may have an atypical clinical picture, especially those with advanced HIV disease, complicating the diagnosis of pulmonary and extrapulmonary forms of TB disease. Access to a rapid and accurate diagnosis is essential to ensure that TB is effectively treated among people living with HIV.
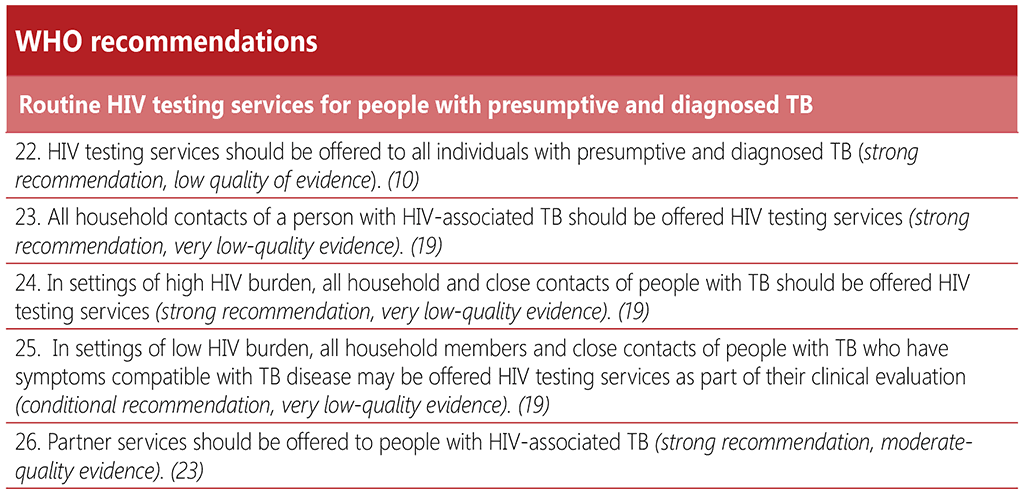
2.1 TB screening
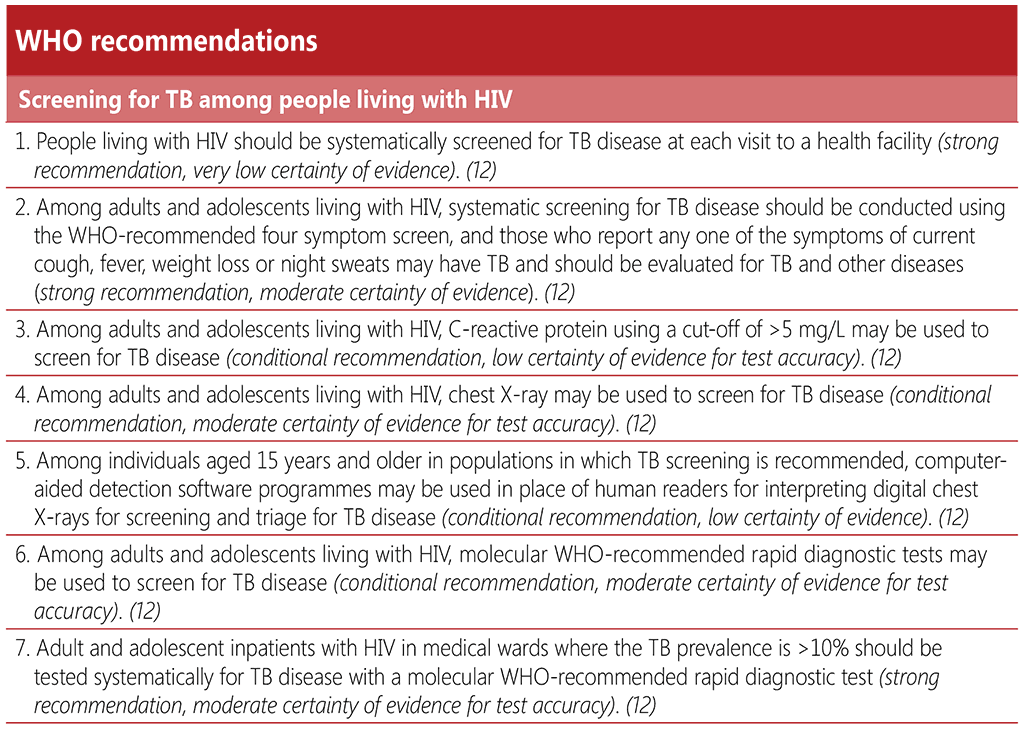
2.1.1 Background
2. Reduce the burden of TB among people living with HIV
Tuberculosis remains the primary cause of HIV-related morbidity and mortality worldwide, despite impressive scale-up of ART. In 2022, an estimated 671 000 (uncertainty interval (UI): 600 000–746 000) people living with HIV developed TB disease, among whom only 426 958 (64%) were diagnosed and notified (1). In the same year, an estimated 167 000 (UI: 139 000–198 000) people living with HIV died from TB, representing 27% of all HIV-related deaths (1).
1.7 Publication, dissemination, implementation, evaluation and expiry
These guidelines are published on the WHO website and can also be freely downloaded from the WHO TB Knowledge Sharing Platform.⁵ Implementation considerations are also reflected in the TB/HIV operational handbook (7).
1.6 Process of consolidating the guidelines
To develop the TB/HIV guidelines, WHO mapped the WHO publications containing recommendations on HIV-associated TB, which had been formulated by the respective GDGs and approved by the WHO Guidelines Review Committee (GRC). All the recommendations included in these guidelines were developed in accordance with the WHO guideline development process, as outlined in the source guidelines. A full list of the source guidelines that were used to consolidate all WHO recommendations and inform the TB/HIV guidelines is provided in Box 1.1.
1.5 Target audience
The TB/HIV guidelines are intended for managers of national TB and HIV programmes at all levels of the health system, managers in the private-for-profit sector, and other decision-makers in the health system. They are also a useful resource for clinicians and other healthcare providers, including community-based and primary care, harm-reduction services and maternal and child health programmes, as well as for relevant line ministries working on HIV-associated TB, such as ministries responsible for prisons or mining services.
Pagination
- Previous page
- Page 13
- Next page

 Feedback
Feedback