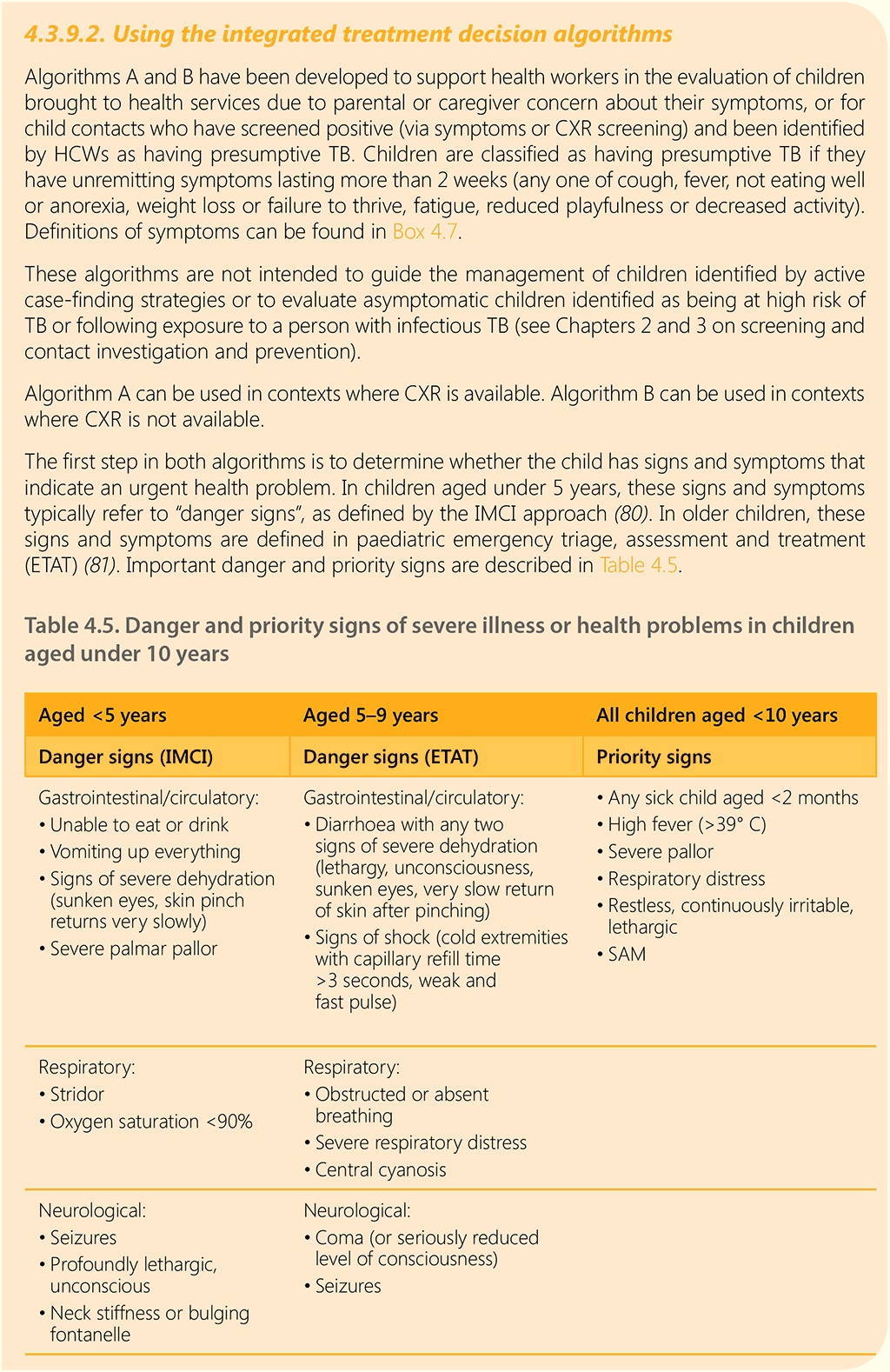
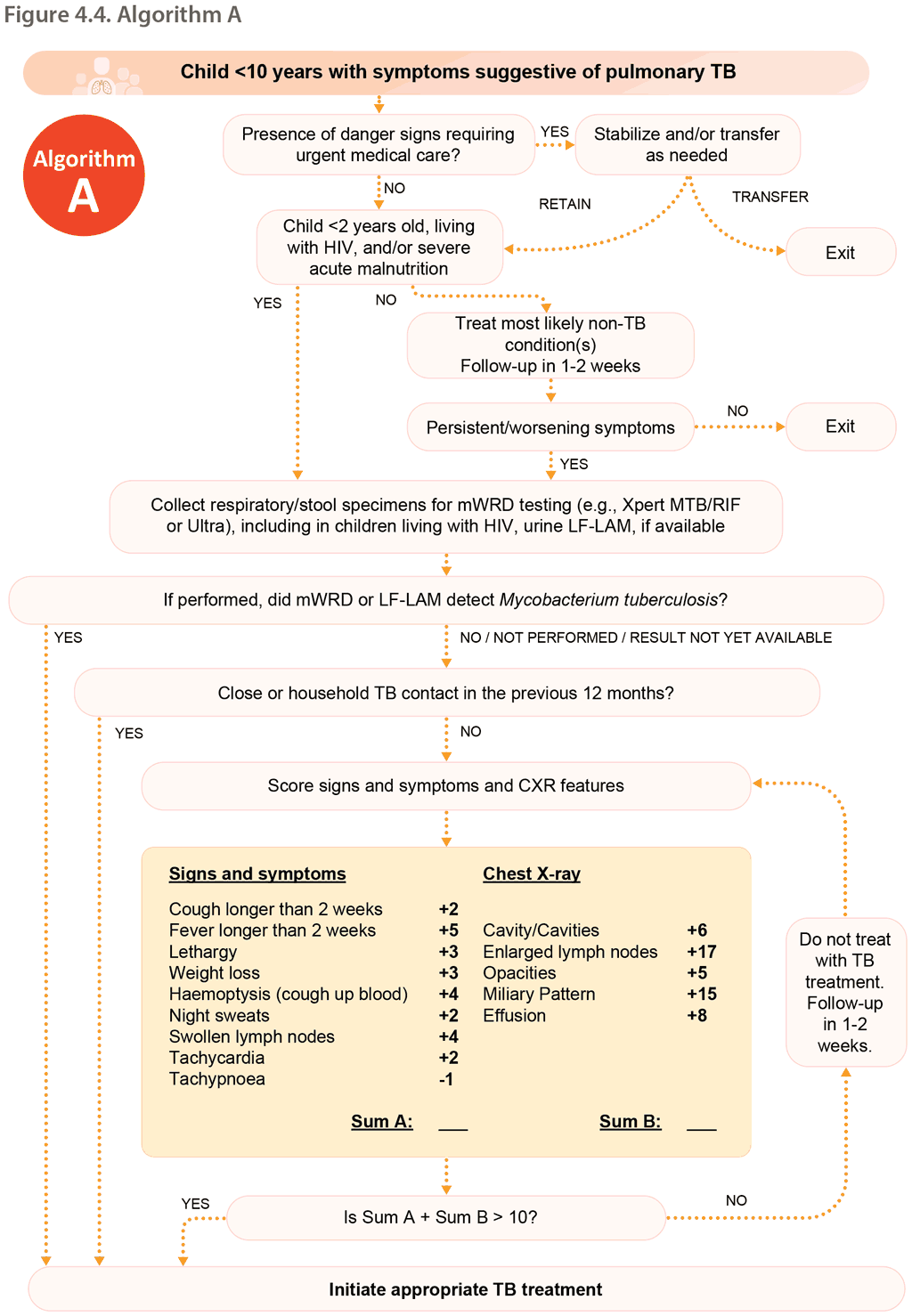
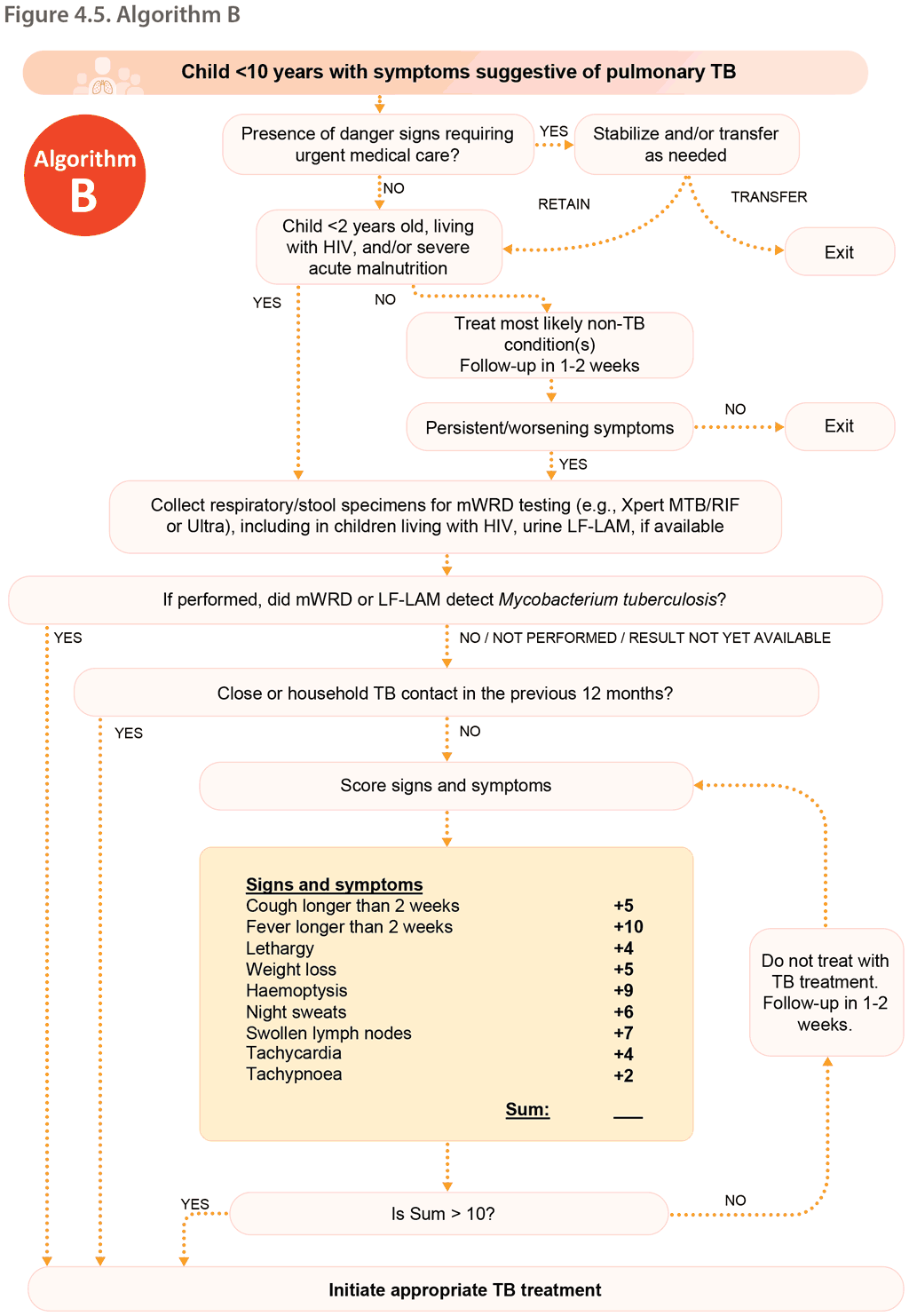
6.2 Decentralized, family-centred, integrated TB services
Decentralization includes the provision of, access to or capacity for child and adolescent TB services at a lower level of the health system than the lowest level where it is currently routinely provided. In most settings, decentralization applies to the district hospital level (first referral level), PHC level or community level.

 Feedback
Feedback