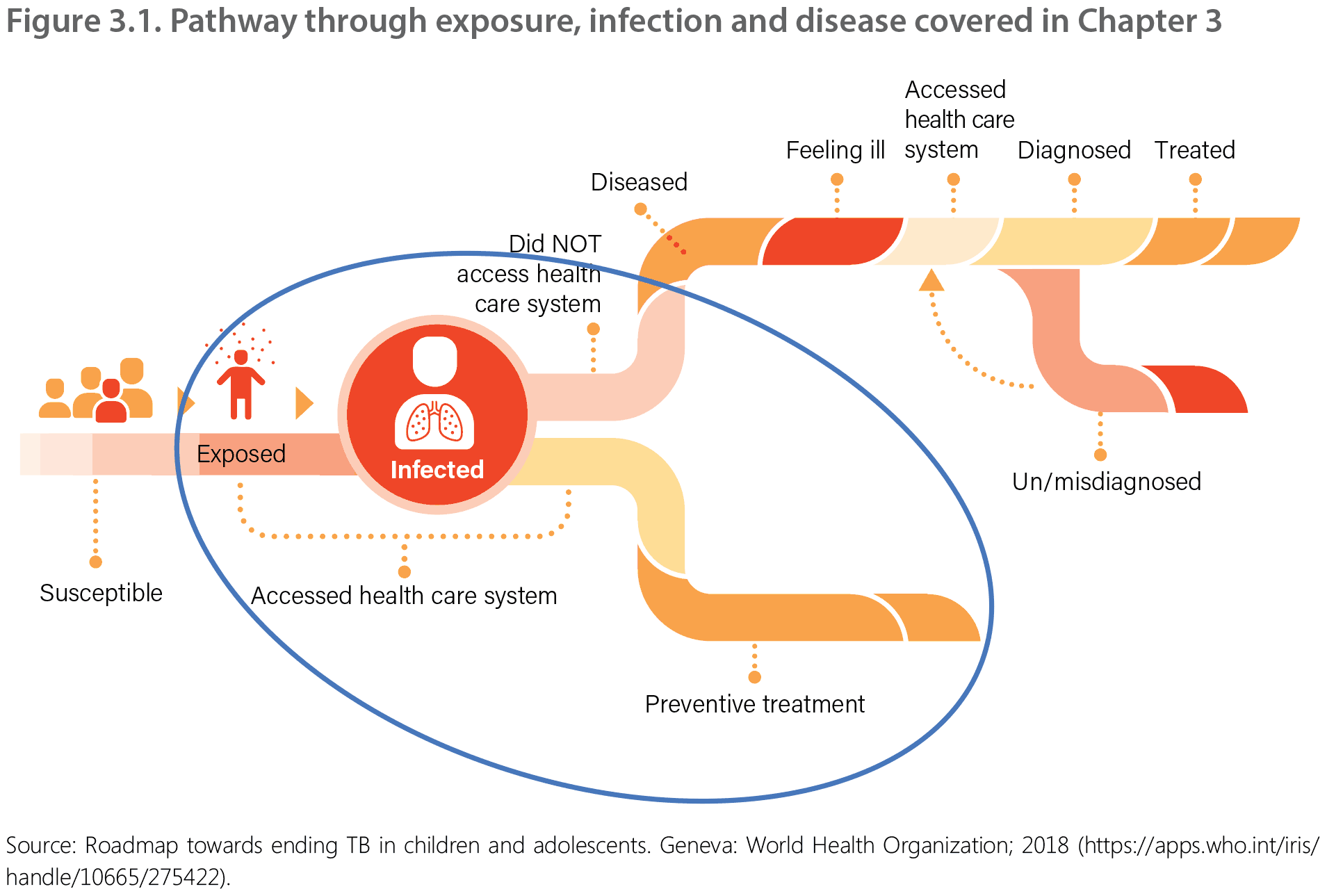
3.3.2.2. Child and adolescent household contacts
Children aged under 5 years who are household contacts of people with bacteriologically confirmed TB have a significantly higher risk of acquiring TB infection and progressing rapidly to TB disease. Children aged under 2 years are also at particularly high risk for severe and disseminated forms of TB with very high risk of morbidity and mortality. TPT is strongly recommended in all TB household contacts aged under 5 years once TB disease is ruled out.

 Feedback
Feedback