Structure of the chapter
The Recommendations part of this chapter has three main sections on elements of TB care and support. The elements covered are:
 TB KaSPar
TB KaSPar
 Feedback
Feedback
The Recommendations part of this chapter has three main sections on elements of TB care and support. The elements covered are:
WHO aims to use the best available evidence on interventions to ensure adequate patient care and support and in order to inform policy decisions made by national TB control programme managers, national policy-makers and medical practitioners in a variety of geographical, economic and social settings.
This chapter of the WHO consolidated guidelines. Module 4: Treatment and care aims to provide a summary of existing WHO recommendations on care and support during tuberculosis treatment.
In addition to summarizing the available evidence, the reviews undertaken for these consolidated guidelines revealed several gaps in current knowledge about critical areas in DR-TB treatment and care. The estimates of effect for patient studies were commonly assigned a low or very low certainty rating, which explains why most of the recommendations in these guidelines are conditional. Some gaps identified in previous TB treatment guidelines (2, 16) persist.
Recommendation 8.1 HCV and MDR/RR-TB treatment

Remarks
Rationale
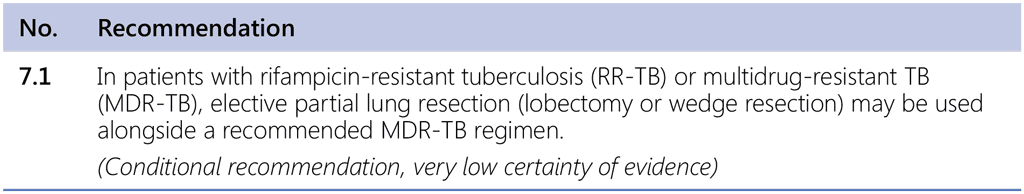
Recommendation 7.1 Surgery for MDR/RR-TB patients
Justification and evidence
The recommendation in this section addresses one PICO question:
Recommendation 6.1 Starting ART

Justification and evidence
The recommendation in this section addresses one PICO question:
People who receive MDR/RR-TB regimens need to be monitored during treatment using relevant clinical and laboratory testing schedules. Response to treatment and toxicity are monitored through regular history taking, physical examination and CXR; special tests (e.g. audiometry, visual acuity tests, peripheral neurological examination and electrocardiography); and laboratory monitoring. Using smear microscopy or culture to assess the conversion of bacteriological status is an important way to assess treatment response.
Recommendations 4.1–4.2 Treatment of Hr-TB

Justification and evidence
The recommendations in this section address one PICO question: