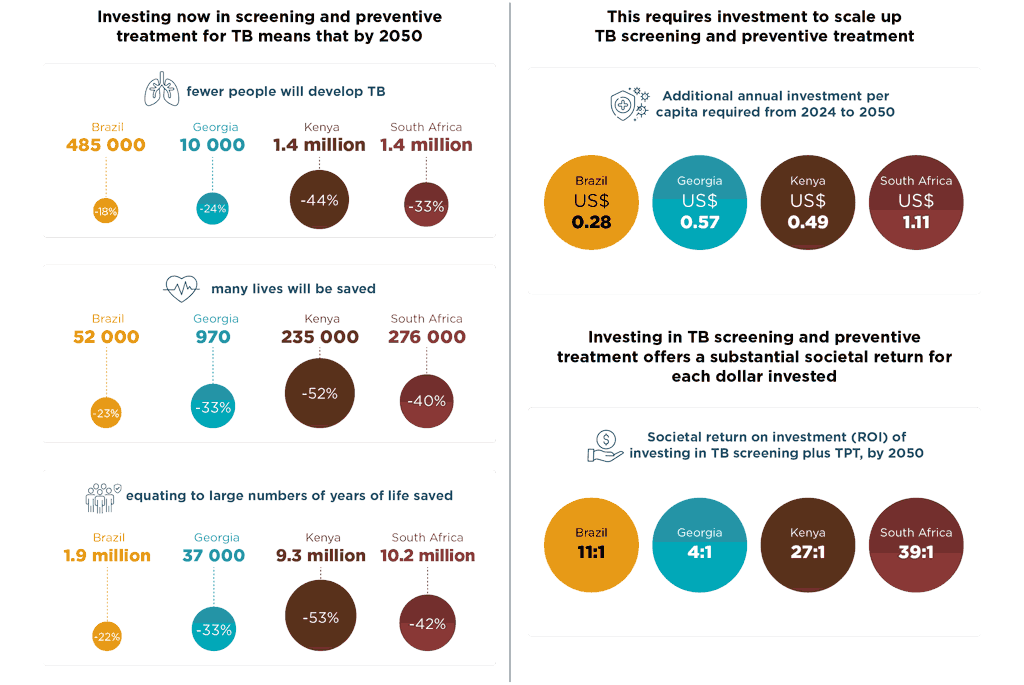
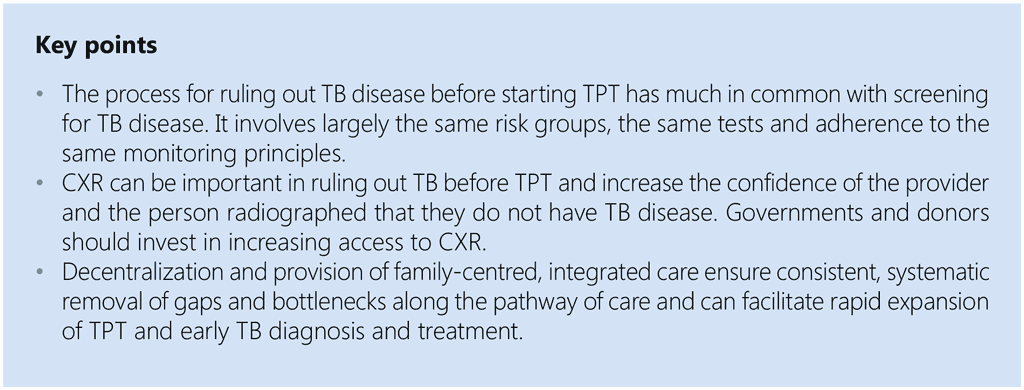
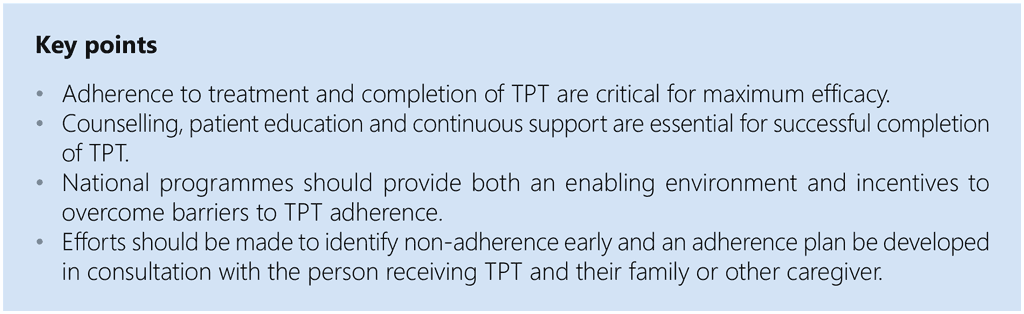
Annex 3. Coordination mechanisms to support PMTPT
Ministries of health should create a national coordinating body and/or national technical expert working group to support national scaling up of PMTPT according to the latest global guidelines. Alternatively, the mandate of an existing body that could serve both management and technical functions could be extended to advise the ministry of health and national TB, HIV and other programmes, thus guiding and supporting governments in fulfilling their national commitments to PMTPT. The technical expert group, although established at national level, should provide advice at all levels.

 Feedback
Feedback