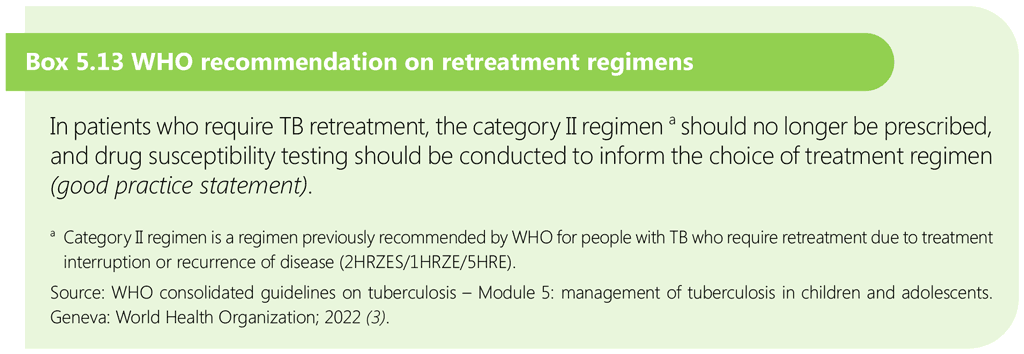
5.3 Treatment of multidrug-resistant and rifampicin-resistant TB in children and adolescents
This section describes a practical approach to the treatment of children with rifampicin-resistant tuberculosis (RR-TB) and MDR-TB (resistant to both rifampicin and isoniazid). It covers identifying children who should be treated for MDR/RR-TB, deciding on the most appropriate treatment regimen, monitoring, and other implementation issues related to treatment.

 Feedback
Feedback