Operational Handbooks
References
1. Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13:e1002152. doi: 10.1371/journal.pmed.1002152.
2. Global tuberculosis report 2020. Geneva: World Health Organization; 2020 (https://apps.who.int/iris/ bitstream/handle/10665/336069/9789240013131-eng.pdf, accessed 19 February 2021).
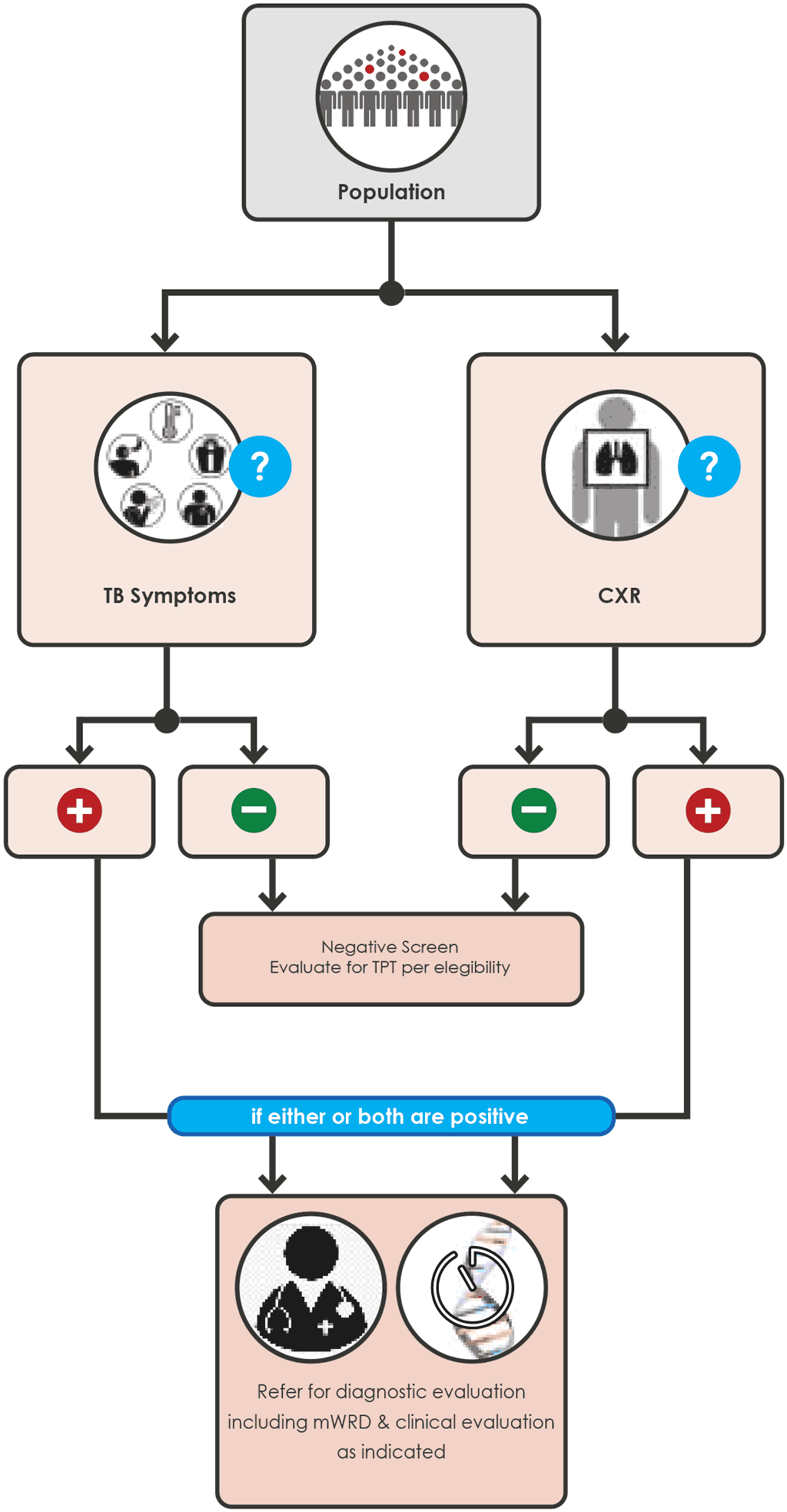
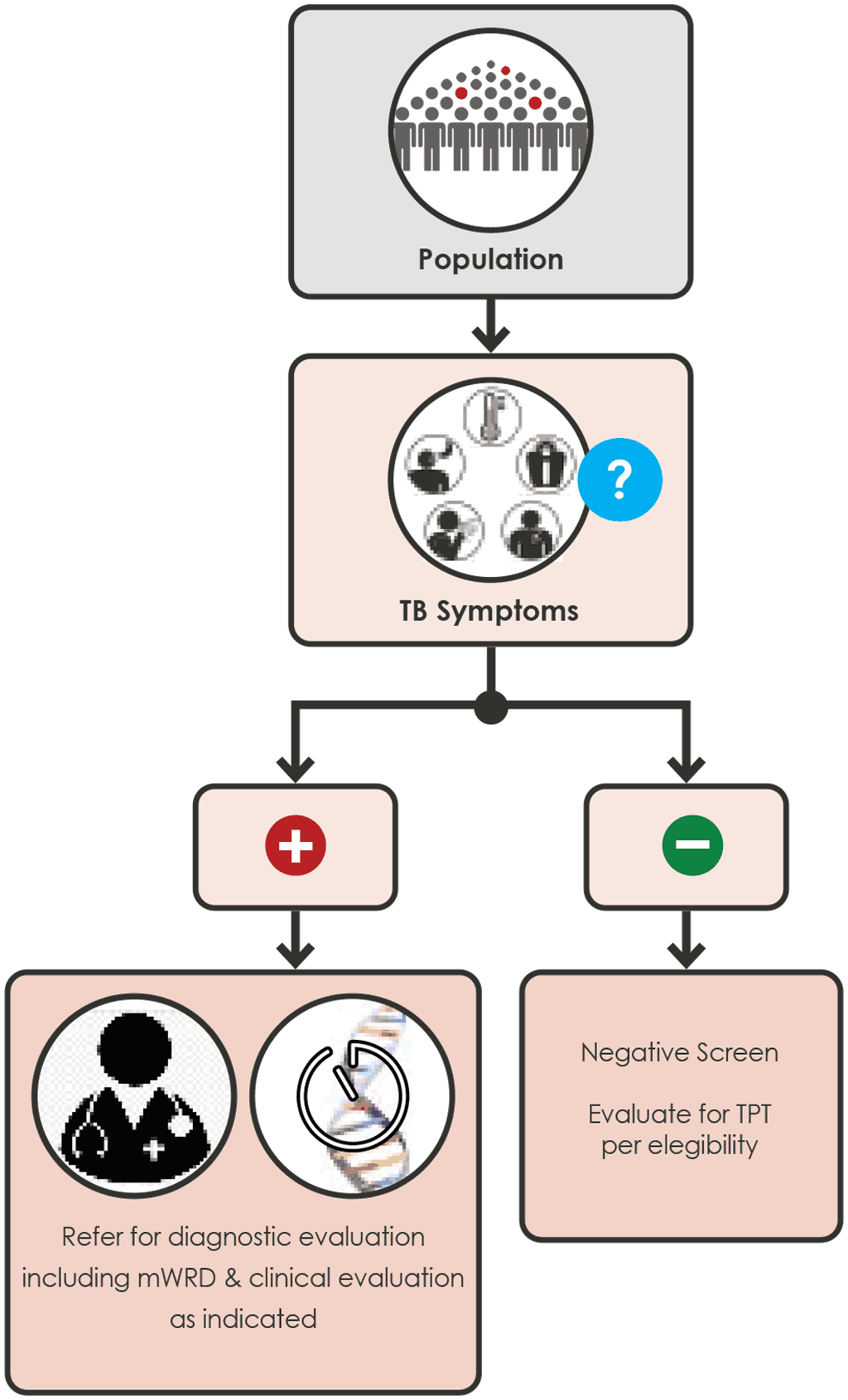
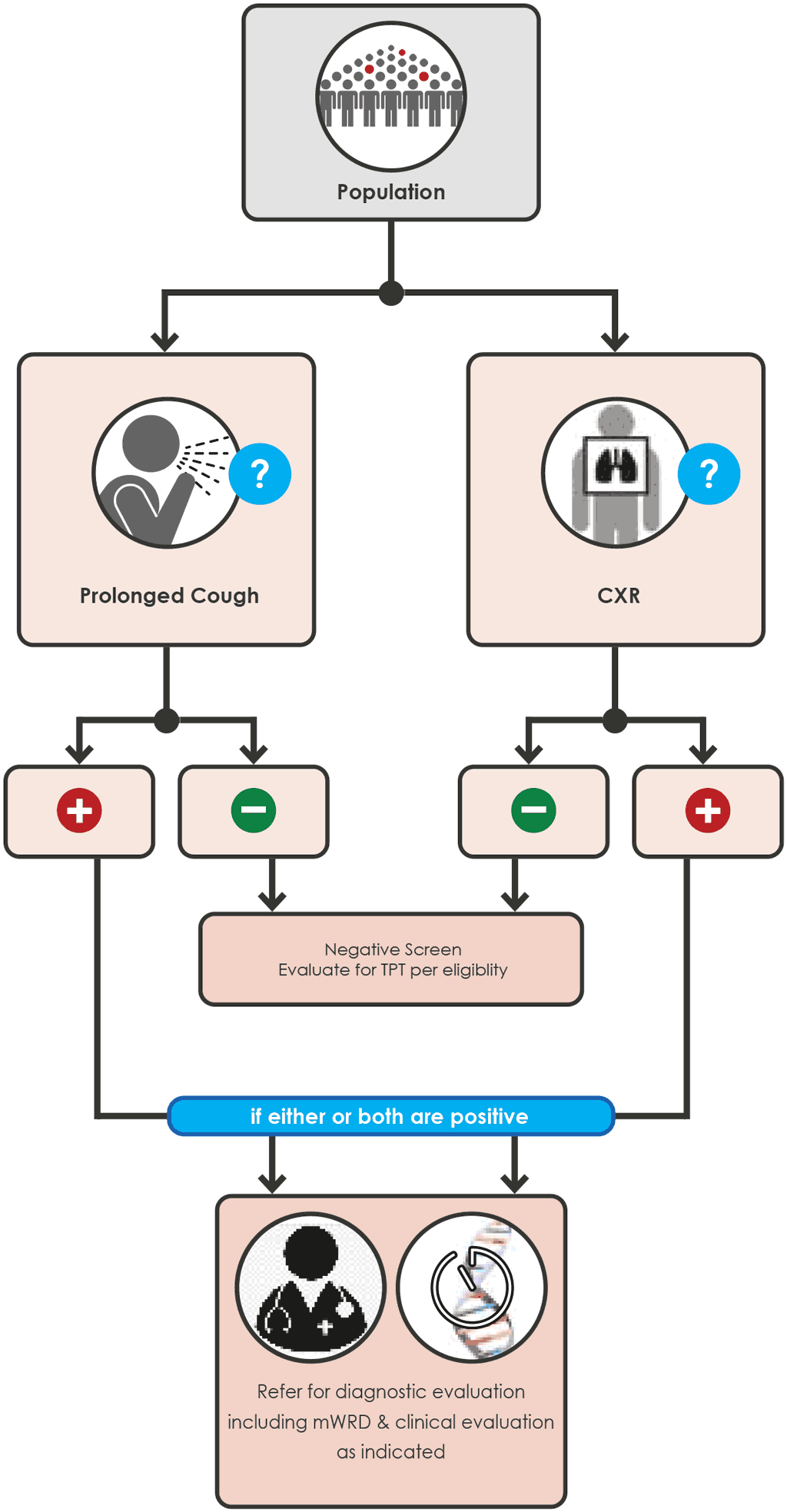
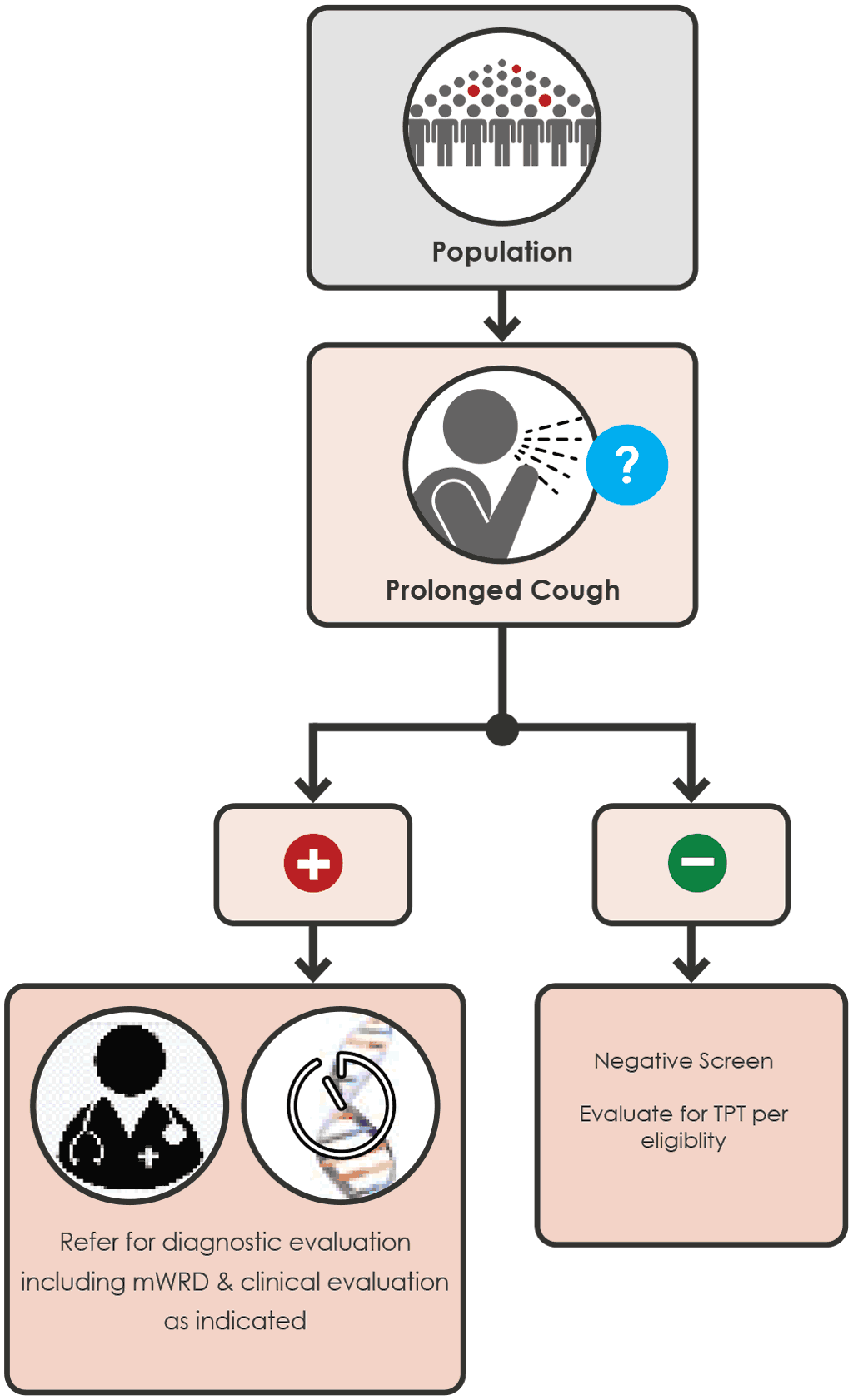
Annex 5 Screening algorithms for children
Illustrations of 6 possible screening algorithms for children (Fig A.5.1 - A.5.5 for child contacts and Fig. A.5.6 for children <10 years living with HIV).
Annex 4 Comparative performance of algorithms for adults and adolescents living with HIV
The tables below contain modelled estimates of the performance and outcomes of the screening algorithms described in Annex 3, when applied to different subpopulations of people living with HIV: outpatients not on ART, outpatients on ART, and inpatients. For each subpopulation, a model is presented of 1,000 persons being screened with a representative TB prevalence. The models were informed by the results of the IPD analysis that was commissioned to evaluate the performance of the W4SS and alternative screening tools in people living with HIV.
Annex 3 Screening algorithms for adults and adolescents living with HIV
Illustrations of 11 possible screening algorithms for people living with HIV.
Annex 1 Screening algorithms for the general population and high-risk groups (not including people living with HIV)
Illustrations of 10 possible algorithms for screening individuals aged 15 years and older among the general population and high-risk groups where screening is recommended.
6.4 Algorithms for screening
Screening algorithms for children are listed in Annex 5.
Children 0 to < 15 years with a close contact with TB
Any of the following screening algorithms can be used:
Fig. A.5.1 Screening with symptoms (page 90)
Fig. A.5.2 Screening with CXR (page 91)
Pagination
- Previous page
- Page 20
- Next page

 Feedback
Feedback