Breadcrumb
- Home
- WHO TB KNOWLEDGE SHARING PLATFORM
- Operational Handbooks
- Module 2: Screening
- Module 2: Systematic screening for tuberculosis disease
- Annex 1 Screening algorithms for the general population and high-risk groups (not including people living with HIV)
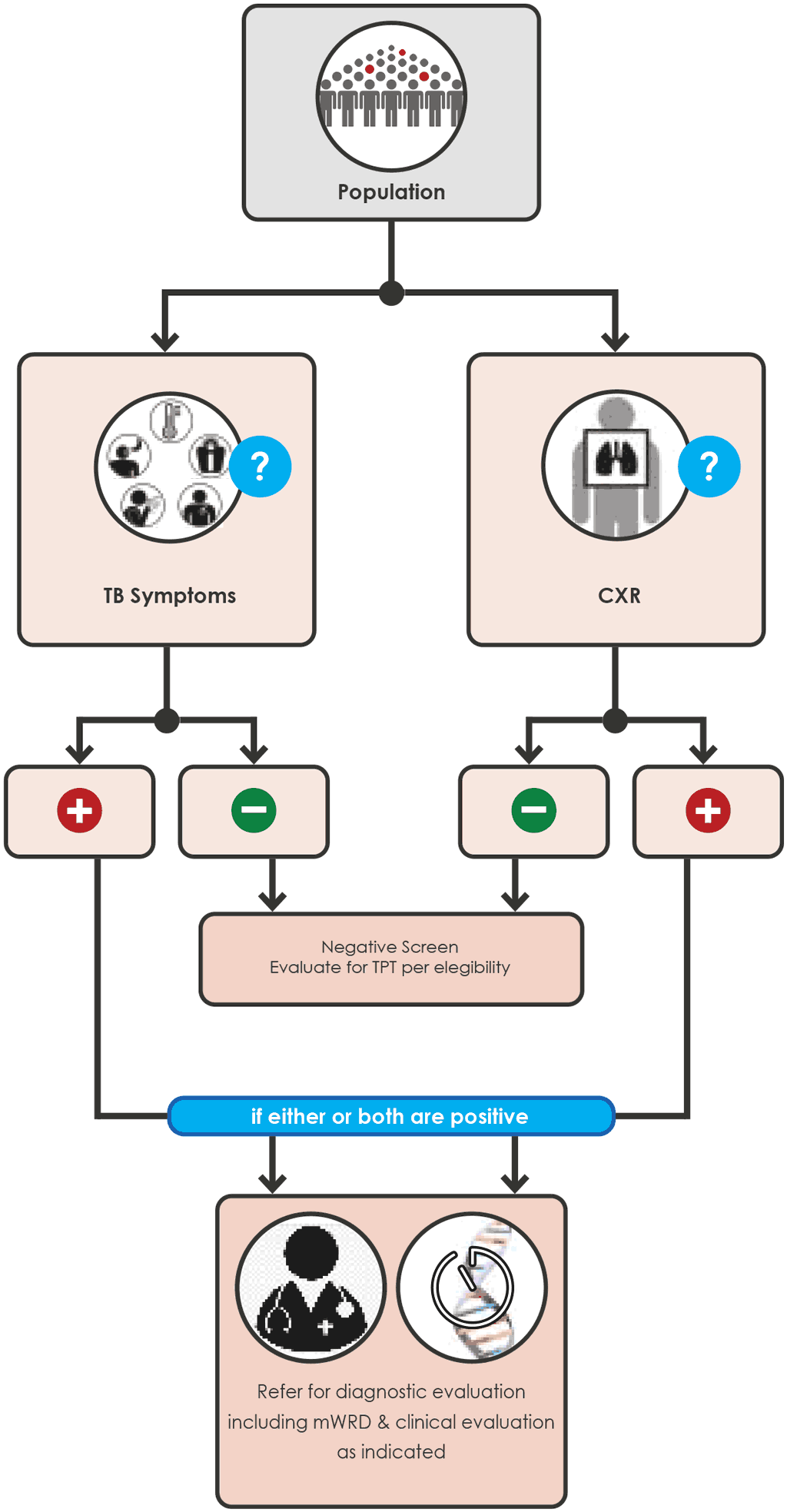
- A.1.6 – Parallel screening with any TB symptom and CXR
A.1.6 – Parallel screening with any TB symptom and CXR
Book navigation
-
Consolidated Guidelines
-
Module 1: Prevention
-
Module 1: TB Preventive Treatment
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
- Introduction
- 1. Recommendations
- 2. Monitoring and evaluation
- 3. Research gaps
- References
- Annex 1. Recommendations in the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment, second edition (2024) and in the previous edition (2020)
- Annex 2. Methods and expert panels
- Annex 3. GRADE summary of evidence tables
- Annex 4. GRADE evidence-to-decision tables
-
Annex 5. Summary of unpublished studies (PICO 10)
- A5.1 Summary of TB CHAMP and V-QUIN clinical trials
- A5.2 Use of fluoroquinolones for TB preventive treatment in contacts of persons with MDR-/RR-TB: A systematic review
- A5.3 Assessing fluoroquinolone (levofloxacin) acceptability among contacts of MDR-TB patients: a qualitative study¹⁰
- A5.4 A survey to explore the programmatic feasibility of levofloxacin (Lfx) TPT for MDR-TB contacts¹¹
- Module 1: TB infection prevention and control
-
Module 1: TB Preventive Treatment
-
Module 2: Screening
-
Module 2: Systematic screening for tuberculosis disease
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
- 1. Introduction
-
2. Recommendations for systematic screening for TB disease in targeted populations
- 2.1 Systematic screening for TB disease among the general population
- 2.2 Systematic screening for TB disease among people with structural risk factors for TB
- 2.3 Systematic screening for TB disease among people living with HIV
- 2.4 Systematic screening for TB disease among household and other close contacts of individuals with TB disease
- 2.5 Systematic screening for TB disease in prisons and other penitentiary institutions
- 2.6 Systematic screening for TB disease among miners and others exposed to silica dust
- 2.7 Systematic screening for TB disease among people attending health care services who have clinical risk factors for TB
-
3. Recommendations for tools for systematic screening for TB disease
- 3.1 Tools for screening for TB disease among the general population and high-risk groups
- 3.2 Use of computer-aided detection software for automated reading of digital chest radiographs
-
3.3 Tools for screening for TB disease among people living with HIV
-
3.3.1 Summary of the evidence and rationale
- 3.3.1.1 WHO-recommended four-symptom screen
- 3.3.1.2 C-reactive protein
- 3.3.1.3 Chest radiography
- 3.3.1.4 Molecular WHO-recommended rapid diagnostic tests for medical inpatients living with HIV in settings with a high TB burden
- 3.3.1.5 Molecular WHO-recommended rapid diagnostic tests for all other people living with HIV
- 3.3.2 Implementation considerations for all tools for screening people living with HIV
-
3.3.1 Summary of the evidence and rationale
- 3.4 Tools for systematic screening for TB disease among children and adolescents
- 4. Monitoring and evaluation
- 5. Research gaps
- 6. References
- Supplementary Table
- Web annexes
-
Module 2: Systematic screening for tuberculosis disease
-
Module 3: Diagnosis
-
Module 3: Diagnosis
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
- 1. Introduction
-
2. Recommendations for diagnosis of TB disease
- 2.1. Initial diagnostic tests for diagnosis of TB with drug-resistance detection
- 2.2. Initial diagnostic tests for diagnosis of TB without drug-resistance detection
- 2.3. Concurrent use of initial diagnostic tests for diagnosis of TB in People living with HIV and children
- 2.4. Follow-on diagnostic tests for detection of additional drug-resistance after TB confirmation
- 2.5. References
- 3. Recommendations for diagnosis of TB infection
- Annex 1. Guideline development methods
- Annex 2. Conflict of interest assessment for Guideline Development Group and External Review Group members
- Annex 3. GDG members expertise, region, gender
-
Module 3: Diagnosis
-
Module 4: Treatment
-
Module 4: treatment and care
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
- Chapter 1. Drug-susceptible TB treatment
-
Chapter 2. Drug-resistant TB treatment
- Introduction
-
Recommendations
- 1. Treatment of drug-resistant TB using 6-month regimens
- 2. Treatment of drug-resistant TB using 9-month regimens
- 3. Treatment of drug-resistant TB using longer regimens
- 4. Treatment of rifampicin-susceptible and isoniazid-resistant TB (Hr-TB)
- 5. Monitoring and management strategies for MDR/RR-TB treatment
- 6. Starting antiretroviral therapy in patients on MDR/RR-TB regimens
- 7. Surgery for patients on MDR/RR-TB treatment
- 8. Hepatitis C virus (HCV) and MDR/RR-TB treatment co-administration
- Research gaps
- References (Chapter 2)
- Chapter 3. Tuberculosis care and support
-
Annex 1. Summary of recommendations
- Annex 1.1. Summary of changes to the World Health Organization (WHO) treatment recommendations for drug-susceptible TB (DS-TB) between 2010 and the update and consolidation in 2025
- Annex 1.2. Summary of changes to the World Health Organization (WHO) treatment recommendations for multidrug-resistant or rifampicin-resistant TB (MDR/RR-TB) between 2019 and the update and consolidation in 2025
- Annex 1.3. Summary of changes to the World Health Organization (WHO) treatment recommendations for Tuberculosis care and support between 2011 and the update and consolidation in 2025
- Annex 2. Methods and expert panels
- Annex 3. Declarations of interest
- Annex 4. PICO questions
- Annex 5. GRADE evidence profiles and evidence-to-decision tables
- Annex 6. Statistical analysis plans
- Annex 7. Reports of the systematic reviews for Tuberculosis care and support
-
Module 4: treatment and care
-
Module 5: Children and adolescents
-
Module 5: Management of tuberculosis in children and adolescents
- Acknowledgements
- Abbreviations
- Definitions
- Executive summary
-
1. Introduction
- 1.1. Background
- 1.2. Rationale for the development of the 2022 consolidated guidelines
- 1.3. Objectives of the 2022 consolidated guidelines
- 1.4. Target audience
- 1.5. WHO recommendations relevant to the management of TB in children and adolescents
- 1.6. Scope of the guideline update
- 1.7. Publication, dissemination, evaluation and expiry
- 1.8. Document structure
- 2. TB screening and contact investigation
- 3. Prevention of TB
-
4. Diagnostic approaches for TB in children and adolescents
- 4.1. The use of the Xpert MTB/RIF Ultra assay in gastric aspirate and stool specimens for the diagnosis of pulmonary TB and rifampicin resistance
- 4.2. Treatment decision algorithms for the diagnosis of pulmonary TB in children aged below 10 years of age
- 4.3. Consolidated recommendations on TB diagnostics and diagnostic approaches relevant to children and adolescents
- 5. Treatment of TB disease in children and adolescents
- 6. Models of TB care for case detection and provision of TPT in children and adolescents
- 7. Special situations
- 8. Research priorities
- 9. References
- Annex 1. WHO recommendations incorporated in the guidelines on the management of TB in children and adolescents
- Annex 2. Supplementary table
-
Module 5: Management of tuberculosis in children and adolescents
-
Module 6: Comorbidities
-
Module 6: Tuberculosis and comorbidities
- Introduction
-
HIV
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
- 1. HIV: introduction
- 2. Reduce the burden of TB among people living with HIV
- 3. Reduce the burden of HIV among people with presumptive or diagnosed TB
- 4. Monitoring and evaluation
- 5. Research gaps
- References
- Annex 1. Current methodology for WHO guideline development
- Annex 2. Summary of changes to recommendations
-
Undernutrition
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
- 1. Undernutrition: introduction
-
2. Recommendations
- 2.1 Nutritional assessment and counselling in people with TB and their household contacts
- 2.2 Nutritional interventions and food assistance
- 2.3 Micronutrient supplementation in people with TB
- 2.4 TB screening for people with undernutrition and people with structural risk factors such as food insecurity
- 3. Monitoring and evaluation
- 4. Research gaps
- Supplementary table: summary of changes to recommendations
- References
- Web Annexes
-
Module 6: Tuberculosis and comorbidities
-
Module 1: Prevention
-
Operational Handbooks
-
Module 1: Prevention
-
Module 1: TB preventive treatment
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- 1. Introduction
- 2. Identifying populations for TB preventive treatment
- 3. Screening for TB and ruling out TB disease before TB preventive treatment
- 4. Testing for TB infection
- 5. TB preventive treatment
- 6. Safety and management of adverse drug reactions in TB preventive treatment
- 7. Supporting people in adhering to and completing TB preventive treatment
- 8. Monitoring and evaluation
- 9. Ethics and TB preventive treatment
- References
-
Annexes
- Annex 1. Investment case for TB screening and preventive treatment
- Annex 2. Messages for different stakeholders
- Annex 3. Coordination mechanisms to support PMTPT
- Annex 4. Costing considerations for PMTPT
- Annex 5. Checklist for PMTPT components in reviews of national programmes
- Annex 6. Variables to be collected for TB contact evaluation
- Web annexes
-
Module 1: TB laboratory biosafety
- Executive summary
- Participants in the guideline development process
- Abbreviations
- Introduction
- 1. Risk assessment and the classification of tb laboratories
- 2. Essential biosafety measures for tb laboratories
- 3. Low-risk tb laboratories
- 4. Moderate-risk tb laboratories
- 5. High-risk tb laboratories (tb-containment laboratories)
- 6. Safety equipment
- 7. Personal protective equipment and clothing
- 8. Plans for emergency preparedness and response
- 9. References
- 10. Annex
-
Module 1: Infection prevention and control
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- 1. Introduction
- 2. Administrative controls
- 3. Environmental controls
- 4. Respiratory protection
- 5. TB IPC in special situations
- 6. Monitoring and evaluation
- References
-
Annexes
- Annex 1. Data elements for monitoring implementation of tuberculosis infection prevention and control
- Annex 2. Facility tuberculosis risk assessment tool
- Annex 3. Example of an outline of facility tuberculosis infection prevention and control plan
- Annex 4. Health care worker tuberculosis screening form
- Annex 5. Health care worker TB screening register
- Annex 6. Sample posters for health education
- Annex 7. How to choose upper-room germicidal ultraviolet light fixtures
- Annex 8. Choosing a radiometer for measurement of ultraviolet C irradiation
- Annex 9. Checklist for the review of programmatic implementation of tuberculosis infection prevention and control
- Annex 10. Country example: education messages for tuberculosis and for tuberculosis infection prevention and control
-
Module 1: TB preventive treatment
-
Module 2: Screening
-
Module 2: Systematic screening for tuberculosis disease
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Chapter 1. Introduction
-
Chapter 2. The six steps in the planning and implementation cycle
- 2.1 Introduction
- 2.2 Assessing the situation
- 2.3 Setting goals and specific objectives
-
2.4 Identifying and prioritizing risk groups
- 2.4.1 Potential benefits for the individual
- 2.4.2 Potential risks and harms for the individual
- 2.4.3 Potential impact on prevalence and transmission
- 2.4.4 Potential total yield of true TB cases
- 2.4.5 Number needed to screen to detect a person with TB
- 2.4.6 Cost–effectiveness and cost–benefit analyses
- 2.5 Choosing algorithms for screening and diagnosis
- 2.6 Planning, budgeting and implementing
- 2.7 Monitoring, evaluating and modifying the programme
- Chapter 3. Screening tools and algorithms
- Chapter 4. Implementation of CAD technologies in a new setting
- Chapter 5. Screening for tuberculosis disease among adults and adolescents living with HIV
- Chapter 6. Screening for tuberculosis disease in children
- References
-
Annex 1 Screening algorithms for the general population and high-risk groups (not including people living with HIV)
- A.1.1 – Screening with cough
- A.1.2 – Parallel screening with cough and CXR
- A.1.3 – Sequential positive serial screening with cough and CXR
- A.1.4 – Sequential negative serial screening with cough and CXR
- A.1.5 – Screening with any TB symptom
- A.1.6 – Parallel screening with any TB symptom and CXR
- A.1.7 – Sequential positive serial screening with any TB symptom and CXR
- A.1.8 – Sequential negative serial screening with any TB symptom and CXR
- A.1.9 – Screening with CXR
- A.1.10 – Screening with mWRD
- Annex 2 Comparative performance of algorithms for the general population and high-risk groups (not including people living with HIV)
-
Annex 3 Screening algorithms for adults and adolescents living with HIV
- A.3.1 – W4SS single screening algorithm
- A.3.2 – CRP single screening algorithm
- A.3.3 – CXR single screening algorithm
- A.3.4 – Parallel screening algorithm with W4SS and CRP
- A.3.5 – Sequential positive screening algorithm with W4SS and CRP
- A.3.6 – Sequential negative screening algorithm with W4SS and CRP
- A.3.7 – Parallel screening algorithm with W4SS and CXR
- A.3.8 – Sequential positive screening algorithm with W4SS and CXR
- A.3.9 – Sequential negative screening algorithm with W4SS and CXR
- A.3.10 – mWRD single screening algorithm for medical inpatients in settings with TB prevalence > 10%
- A.3.11 – mWRD single screening algorithm for people living with HIV
- Annex 4 Comparative performance of algorithms for adults and adolescents living with HIV
-
Annex 5 Screening algorithms for children
- A.5.1 – Screening with symptoms
- A.5.2 – Screening with CXR
- A.5.3 – Parallel screening with symptoms and CXR
- A.5.4 – Sequential positive serial screening with symptoms and CXR
- A.5.5 – Sequential negative serial screening with symptoms and CXR
- A.5.6 – Screening with symptoms (for children living with HIV < 10 years)
-
Module 2: Systematic screening for tuberculosis disease
-
Module 3: Diagnosis
-
Module 3: Diagnosis
- Acknowledgements
- Abbreviations and acronyms
- 1. Introduction
-
2. TB tests with WHO recommendations
- 2.1 Conventional tests for the diagnosis of TB
- 2.2 Initial tests for diagnosis of TB with drug resistance detection
- 2.3 Initial tests for diagnosis of TB without drug resistance detection
- 2.4 Follow-on diagnostic tests for detection of additional drug resistance
- 2.5 Phenotypic and genotypic drug resistance testing methods
- 2.6 Tests for TB infection
- 2.7 Tests WHO recommends against using or recommends limited usage
-
3. Strategies and considerations for diagnostic testing
- 3.1 Epidemiological considerations
- 3.2 Pretest probability and test accuracy considerations
- 3.3 Planning for and implementing quality-assured TB testing services
- 3.4 Concurrent testing to improve case detection in children and in people (of all ages) living with HIV
- 3.5 Testing for TB infection
- 3.6 Multidisease testing considerations
- 4. Placement of diagnostic tests in the tiered laboratory network
-
5. Steps and processes for implementing a new diagnostic test
- 5.1 Area 1 – Policies, budgeting and planning
- 5.2 Area 2 – Regulatory issues
- 5.3 Area 3 – Equipment
- 5.4 Area 4 – Supply chain
- 5.5 Area 5 – Procedures
- 5.6 Area 6 – Digital data
- 5.7 Area 7 – Quality assurance, control and assessment
- 5.8 Area 8 – Recording and reporting
- 5.9 Area 9 – Human resource training and competency assessment
- 5.10 Area 10 – Monitoring and evaluation
-
6. Model algorithms
- 6.1 Implementing a new diagnostic algorithm
- 6.2 The cascade of the four model algorithms
- 6.3 Algorithm 1 – WRDs as initial diagnostic tests for TB
- 6.4 Algorithm 2 – DST for second-line drugs for people with MDR/RR-TB
- 6.5 Algorithm 3 – Follow-on testing for individuals with RIF-susceptible TB at risk of resistance to other drugs
- 6.6 Discordant results
- 6.7 Algorithm 4 – Testing for TB infection
- 6.8 Illustrative algorithm combinations
- References⁷
- Annex 1. Budgetary considerations for implementing a new diagnostic test
- Annex 2. Drug susceptibility testing methods and critical concentrations
- Annex 3. Implementation of next-generation sequencing technologies
- Annex 4. Skin tests for tuberculosis infection – detailed description
- Web Annexes
-
Module 3: Diagnosis
-
Module 4: Treatment
-
Module 4: treatment and care
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
-
Chapter 1 Drug-susceptible TB treatment
- 1. Introduction
- 2. Key considerations in DS-TB treatment
- 3. Treatment of DS-TB using the 6-month regimen
- 4. Treatment of DS-TB using the 4-month 2HPMZ/2HPM regimen
- 5. Treatment of DS-TB using the 4-month 2HRZ(E)/2HR regimen
- 6. Treatment of DS-TB in people living with HIV
- 7. Treatment of extrapulmonary TB
- 8. Treatment of DS-TB in special situations
- 9. Monitoring treatment response
- 10. Outcome definitions
- References (Chapter 1)
-
Chapter 2 Drug-resistant TB treatment
- 1. Introduction
- 2. WHO recommendations on DR-TB treatment
- 3. Key considerations in DR-TB treatment
- 4. Treatment of DR-TB using 6-month regimens
- 5. Treatment of drug-resistant TB using 9-month regimens
- 6. Treatment of drug-resistant TB using longer regimens
- 7. Regimen for rifampicin-susceptible and isoniazid-resistant TB
- 8. Adjuncts to DR-TB treatment and comorbidities
- 9. Programmatic implementation of DR-TB regimens
- References (Chapter 2)
- Chapter 3 Tuberculosis care and support
- Annex 1. Tuberculosis medicine information sheets
- Annex 2. Monitoring and management of adverse events in treatment of drug-resistant tuberculosis
- Annex 3. Active TB drug-safety monitoring and management for treatment of drug-resistant tuberculosis
- Annex 4. Dosing of medicines used in TB regimens, adults and children
-
Module 4: treatment and care
-
Module 5: Children and adolescents
-
Module 5: Management of tuberculosis in children and adolescents
- Acknowledgments
- Abbreviations
- Definitions
- 1. Introduction
- 2. TB screening and contact investigation
-
3. Prevention of TB in children and adolescents
- 3.1 Introduction
- 3.2 BCG vaccination
-
3.3 TB preventive treatment
- 3.3.1. Introduction
- 3.3.2. Target groups for TB preventive treatment
-
3.3.3. Ruling out TB disease before starting TB preventive treatment
- 3.3.3.1. HIV-negative household and close contacts of a person with pulmonary TB: infants and children aged under 5 years
- 3.3.3.2. HIV-negative household and close contacts of a person with pulmonary TB: children and adolescents aged 5 years and over
- 3.3.3.3. Children and adolescents living with HIV
- 3.3.4. Testing for TB infection
- 3.3.5. Options for TB preventive treatment regimens: drug-susceptible TB
- 3.3.6. Options for TB preventive treatment regimens: drug-resistant TB
- 3.3.7. Follow-up of children and adolescents on TB preventive treatment
- 3.3.8. Adherence to TB preventive treatment
- 3.3.9. Other issues related to TB preventive treatment in children and adolescents
- 3.4 TB infection prevention and control
- Key messages
-
4. TB diagnostic approaches for children and adolescents
- 4.1 Introduction
- 4.2 Diagnosing TB in children and adolescents
-
4.3 Diagnostic approaches: pulmonary TB
- 4.3.1. Typical symptoms of pulmonary TB
- 4.3.2. History of TB contact
- 4.3.3. Clinical examination
- 4.3.4. Atypical clinical presentations of children with pulmonary TB
- 4.3.5. Bacteriological confirmation
- 4.3.6. Testing for TB infection
- 4.3.7. Role of chest X-ray
- 4.3.8. HIV testing
- 4.3.9. Integrated treatment decision algorithms for pulmonary TB in children
- 4.4 Diagnostic approaches: extrapulmonary TB
- 4.5 Disease severity
- 4.6 Diagnostic approaches: drug-resistant TB
- Key messages
-
5. Treatment of drug-susceptible and drug-resistant pulmonary and extrapulmonary TB in children and adolescents
- 5.1 Introduction
-
5.2 Treatment of drug-susceptible TB in children and adolescents
- 5.2.1. Principles of TB management
- 5.2.2. Treatment of pulmonary TB in children and adolescents
- 5.2.3. Recommended regimens for treatment of drug susceptible pulmonary TB in children
- 5.2.4. Implementation considerations
-
5.2.5. Subgroup considerations
- 5.2.5.1. Children with peripheral lymph node TB
- 5.2.5.2. Children and adolescents living with HIV
- 5.2.5.3. Children with severe acute malnutrition
- 5.2.5.4. Children with severe acute pneumonia
- 5.2.5.5. Infants aged under 3 months or weighing less than 3 kg
- 5.2.5.6. Children and adolescents treated for TB in past 2 years
- 5.2.5.7. Children and young adolescents with severe pulmonary TB
- 5.2.6. Treatment of drug-susceptible extrapulmonary TB in children and adolescents
-
5.2.7. Recommended dosing of first-line medicines in children
- 5.2.7.1. Recommended dosages for first-line TB medicines
- 5.2.7.2. Dosage tables and formulations for treatment of drug-susceptible TB in children and adolescents
- 5.2.7.3. Dosing table for the short intensive TB meningitis regimen
- 5.2.7.4. Dosing of first-line medicines in older children and adolescents over 25 kg (excluding the short intensive TB meningitis regimen)
- 5.2.7.5. Pyridoxine supplementation
- 5.2.8. Additional management considerations
- 5.2.9. Nutritional support
- 5.2.10. Management of adverse events from medicines used to treat drug-susceptible TB
- 5.2.11. Treatment adherence
- 5.2.12. Follow-up and monitoring of children and adolescents on TB treatment
- Key messages: treatment of DS-TB
-
5.3 Treatment of multidrug-resistant and rifampicin-resistant TB in children and adolescents
- 5.3.1. Identifying children who should be treated for multidrug-resistant and rifampicin-resistant TB
-
5.3.2. Multidrug-resistant and rifampicin-resistant TB treatment regimens for children and adolescents
- 5.3.2.1. Overview and approach to selecting a treatment regimen
- 5.3.2.2. Shorter all-oral bedaquiline-containing regimen for multidrug-resistant and rifampicin-resistant TB in children
- 5.3.2.3. Longer individualized regimens for children with multidrug-resistant and rifampicin-resistant TB who are not eligible for the standardized all-oral bedaquiline-containing regimen
- 5.3.2.4. Practical approach to designing individualized multidrug-resistant and rifampicin-resistant TB treatment regimens
- 5.3.2.5. Special considerations: TB meningitis
- 5.3.2.6. Special considerations: TB/HIV coinfection
- 5.3.3. Dosing and formulations of second-line TB medicines in children and young adolescents
- 5.3.4. Monitoring of children and adolescents on multidrugresistant and rifampicin-resistant TB treatment
- Key messages: treatment of DR-TB
- 5.4 Practical guidance for assessment and management of post-TB health in children and adolescents
-
6. Models of TB care for children and adolescents
- 6.1 Introduction
-
6.2 Decentralized, family-centred, integrated TB services
-
6.2.1. Implementation considerations
- 6.2.1.1. Stakeholder engagement
- 6.2.1.2. Regulatory framework and policy guidance
- 6.2.1.3. Health workforce
- 6.2.1.4. Treatment support
- 6.2.1.5. Recording and reporting
- 6.2.1.6. Access to diagnostic supplies and child-friendly formulations of TB medicines
- 6.2.1.7. Resource requirements
- 6.2.1.8. Opportunities for integration of TB services into other services
- 6.2.1.9. Socioeconomic impact of TB on children, adolescents and families
- 6.2.1.10. Examples of country experiences in development of family-centred, decentralized and integrated TB services for children and adolescents
-
6.2.1. Implementation considerations
- 6.3 Private-sector involvement in care for children and adolescents with TB
- 6.4 Differentiated TB service delivery
- 6.5 TB and health emergencies
- Key messages
-
7. Special situations
-
7.1 Management of TB in children and adolescents living with HIV
- 7.1.1. Introduction
- 7.1.2. TB screening in children and adolescents living with HIV
- 7.1.3. Prevention of TB in children and adolescents living with HIV
- 7.1.4. Diagnosis of TB in children and adolescents living with HIV
- 7.1.5. Treatment of TB in children and adolescents living with HIV
- 7.1.6. Co-trimoxazole preventive therapy
- 7.1.7. Antiretroviral therapy
- 7.1.8. Immune reconstitution inflammatory syndrome
- 7.2 TB in pregnancy and management of newborns of mothers with TB disease
- 7.3 Palliative care for children and adolescents with TB
-
7.4 Care for adolescents with or at risk of TB
- 7.4.1. Physical and mental health
- 7.4.2. Connectedness and positive contribution to society
- 7.4.3. Safety and a supportive environment
- 7.4.4. Learning, competence, education, skills and employability
- 7.4.5. Agency and resilience
- 7.4.6. Substance abuse and late presentation to care
- 7.4.7. Poor adherence
- 7.4.8. Making TB services more adolescent-friendly
- 7.5 TB in children with severe acute pneumonia
- 7.6 Management of children with TB and malnutrition
- Key messages
-
7.1 Management of TB in children and adolescents living with HIV
- 8. References
- Annex 1. Selected resources on child and adolescent TB
- Annex 2. Tuberculin skin testing: administration, reading and interpretation
- Annex 3. Sample collection methods
- Annex 4. Standard operating procedures for sample collection methods
- Annex 5. Treatment decision algorithms
- Annex 6. Dosing of medicines used in second-line multidrug-resistant TB regimens by weight band (below 46 kg)
- Annex 7. Overview of options for neurocognitive and functional testing at end of treatment for TB meningitis
-
Module 5: Management of tuberculosis in children and adolescents
-
Module 6: Comorbidities
-
Module 6: Tuberculosis and comorbidities
- Introduction
-
Mental health conditions and substance use disorders
- Acknowledgements
- Abbreviations
- Definitions
- 1. Mental health conditions and substance use disorders: background and rationale
- 2. People-centred care for mental health conditions and substance use disorders in people affected by TB
- 3. Identifying and managing care for mental health conditions and substance use disorders in people affected by TB
- 4. Special considerations
- References
- Annex. WHO resources for mental and substance use disorders
-
HIV
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- 1. Background and rationale
-
2. Establish and strengthen collaboration across health programmes and across sectors for delivering people-centred services for HIV-associated TB
- 2.1 Strengthen governance and accountability for TB/HIV collaborative activities
- 2.2 Conduct an analysis of access to quality services for TB and HIV
- 2.3 Coordinate planning and resource mobilization for collaborative action
- 2.4 Implement and scale up people-centred services for HIV-associated TB
- 2.5 Strengthen monitoring, evaluation and research
- 3. Reduce the burden of TB among people living with HIV
- 4. Reduce the burden of HIV among people with TB
- References
- Annex 1. Monitoring and evaluation of collaborative TB/HIV activities
- Annex 2. Methods for algorithms for diagnosis of TB in people living with HIV
- Annex 3. Checklist for TB infection prevention and control
- Annex 4. Package of care for advanced HIV disease
-
Diabetes
- Acknowledgements
- Abbreviations
- Definitions
- References
- 1. Background and rationale
-
2. Establish and strengthen collaboration across health programmes and across sectors for delivering people-centred services for TB and diabetes.
- 2.1 Strengthen governance and accountability for collaborative TB/DM activities.
- 2.2 Conduct an analysis of access to quality services for TB and diabetes.
- 2.3 Coordinate planning and resource mobilization for collaborative action.
- 2.4 Implement and scale up people-centred services for TB and diabetes.
- 2.5 Strengthen monitoring, evaluation and research.
-
3. Reduce the burden of TB among people living with diabetes.
- 3.1 TB screening among people living with diabetes
- 3.2 TB diagnosis among people living with diabetes
- 3.3 TB treatment among people living with diabetes
- 3.4 Glycaemic control to prevent TB among people living with diabetes
- 3.5 TPT for people living with diabetes
- 3.6 TB IPC in clinics that provide diabetes care
- 4. Reduce the burden of diabetes among people with TB.
- References
- Annex 1. Summary of declaration of interests
- Annex 2. Research gaps in the response to TB and diabetes
- Annex 3. Criteria for referral to higher levels of care
- Annex 4. Monitoring and evaluation of collaborative TB/DM activities
- Annex 5. Examples of recording and reporting forms
- Annex 6. A standardized checklist for periodic evaluation of TB infection prevention and control
-
Module 6: Tuberculosis and comorbidities
-
Module 1: Prevention
- Training Catalogue
- TB Drug Dosage Finder

 Feedback
Feedback