Module 3: Diagnosis

 TB KaSPar
TB KaSPar
 Feedback
Feedback

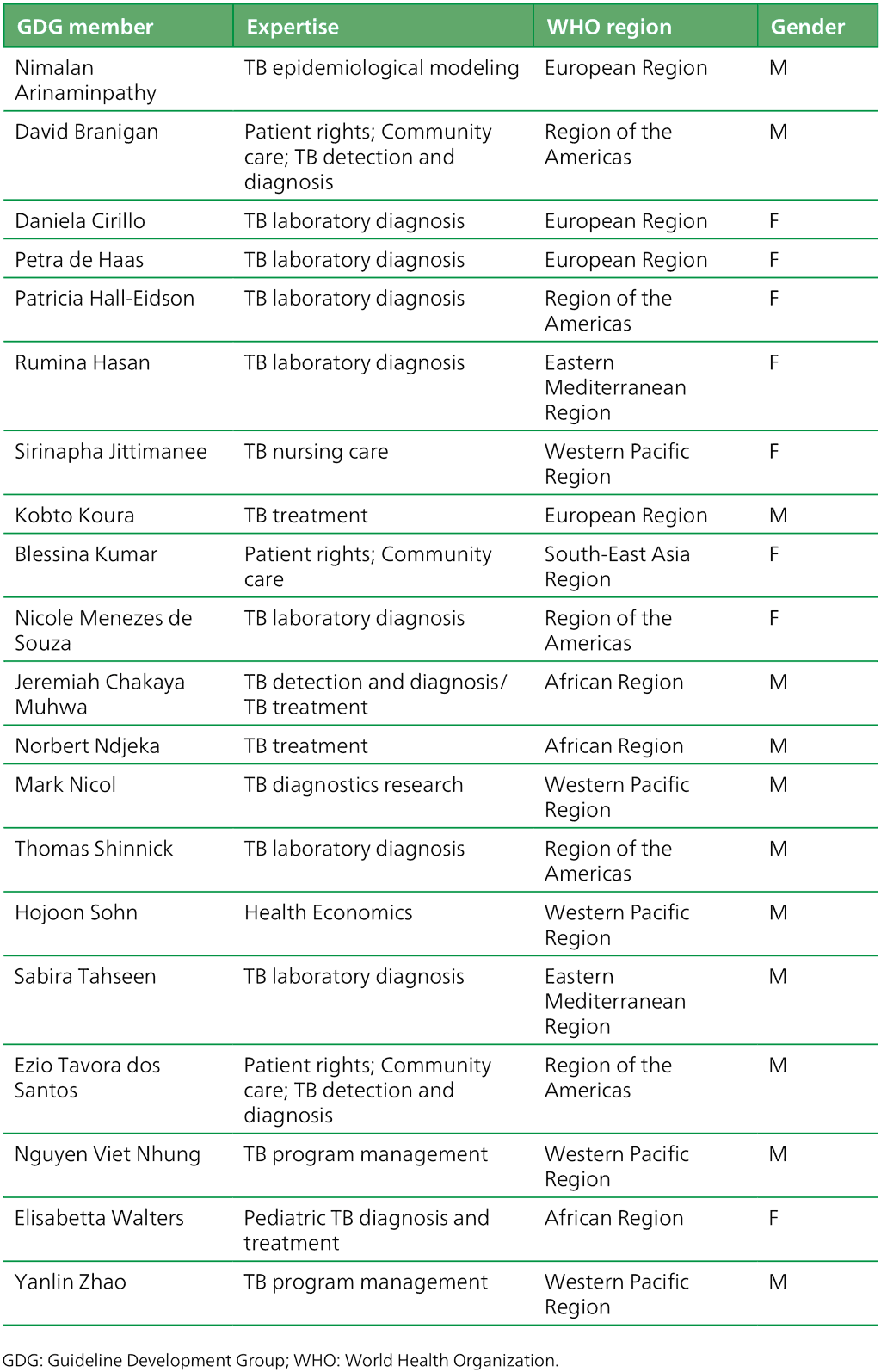
Table A.3.1. Guideline development group members: “Targeted next-generation sequencing” 2–5 May 2023
Before being considered for group membership, each Guideline Development Group (GDG) and External Review Group candidate was required to submit a completed declaration of interest (DOI) form. In addition, a preliminary internet search was performed to identify any obvious public controversies or interests that may lead to compromising situations for the World Health Organization (WHO) and the expert concerned.
To develop new or update existing guidelines for methods and tools to diagnose tuberculosis (TB), the World Health Organization (WHO) Global TB Programme commissions systematic reviews on the performance or use of the tool or method in question. A systematic review provides a summary of the current literature on diagnostic accuracy or user aspects, for the diagnosis of TB or the detection of anti-TB drug resistance in adults or children (or both) with signs and symptoms of TB.
Testing for TB infection increases the certainty that individuals targeted for treatment will benefit from it. However, there is no gold-standard test to diagnose TB infection. Both currently available tests – the TST and IGRAs – are indirect and require a competent immune response to identify people infected with TB. A positive test result by either method is not by itself a reliable indicator of the risk of progression to active disease. This section discusses the evidence and the recommendations for TB infection testing.
Since 2011, the World Health Organization (WHO) has issued recommendations on the use of IGRAs for the diagnosis of TB infection. In 2018, WHO updated the recommendations to stipulate that the TST or IGRAs (or both) can be used to test for TB infection in LMIC. The TST is a widely used point-of-care test that involves intradermal injection of purified protein derivative (PPD), a crude mixture of different mycobacterial antigens, which stimulates a delayed-type hypersensitivity response and causes induration at the injection site within 48–72 hours.