Book traversal links for WHO TB KNOWLEDGE SHARING PLATFORM
Before being considered for group membership, each Guideline Development Group (GDG) and External Review Group candidate was required to submit a completed declaration of interest (DOI) form. In addition, a preliminary internet search was performed to identify any obvious public controversies or interests that may lead to compromising situations for the World Health Organization (WHO) and the expert concerned.
The candidate’s curriculum vitae (CV) and DOI, and information retrieved from the internet, were examined by steering committee members to assess whether there were, or may be, actual or perceived conflicts of interest and, if so, whether a management plan was required. This evaluation process, and resultant management plans, were based on the Guidelines for declaration of interests (WHO experts) (1) and the WHO handbook for guideline development (2nd edition) (2).
Both financial and non-financial interests were considered. A “significant” conflict of interest would include:
- “intellectual bias”, where an individual may have repeatedly and publicly taken a position on an issue under review, which may affect the individual’s objectivity and independence in the global policy development process;
- involvement in research or publication of materials related to issues under review; and
- a financial interest above US$ 5000.
Developers of any assay are never involved in the process of policy development; this is automatically considered a conflict of interest.
Once a determination was made that either no conflict of interest existed, or any conflict of interest could be appropriately managed, and a decision had been made to appoint the candidate, the name and a brief biography of each candidate were published on the WHO website for at least 14 days before the meeting, for public notice and comment.
DOI statements are summarized by the WHO steering committee at the start of the meeting. Selected individuals with intellectual or research involvement were invited as technical resource persons to provide technical input and answer technical questions. These individuals did not participate in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) evaluation process and were excluded from the group discussions when recommendations were developed.
Table A.2.1. Summary of the declarations of interest statements for the GDG members: “Molecular assays intended as initial tests”, 7–18 December 2020
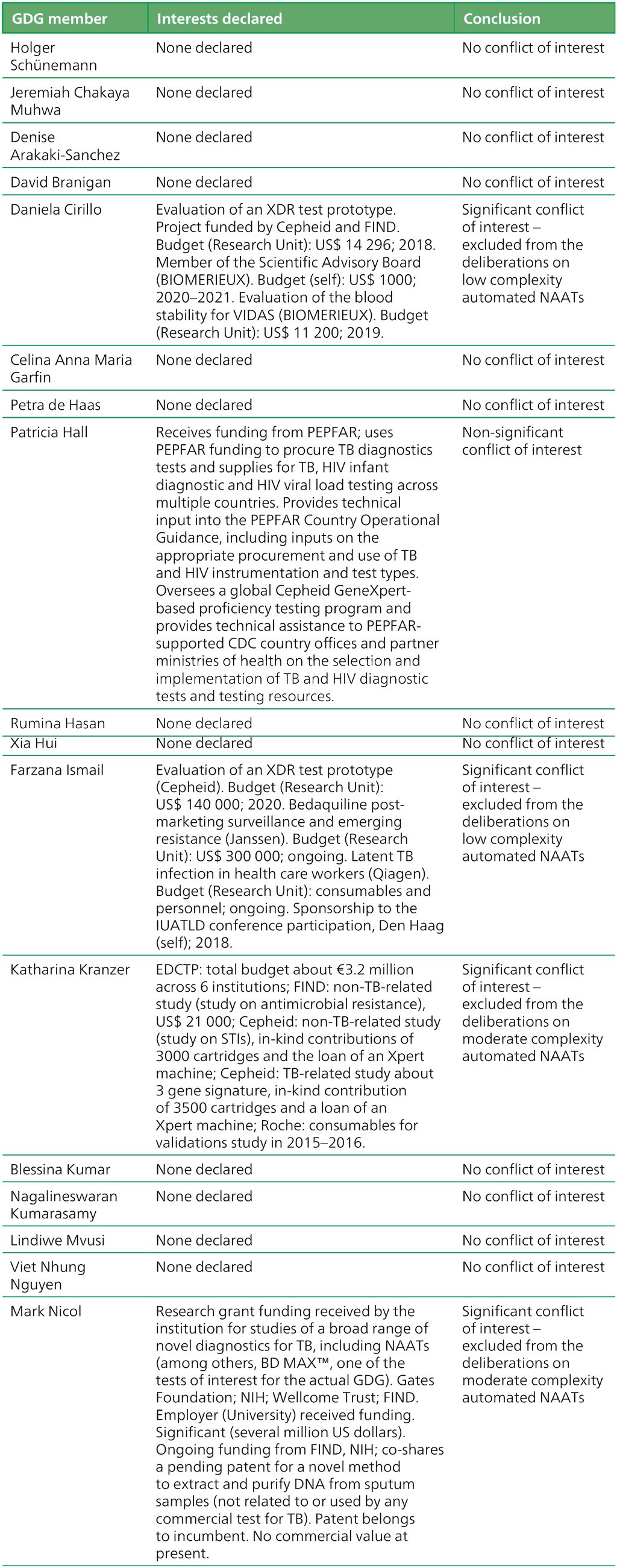
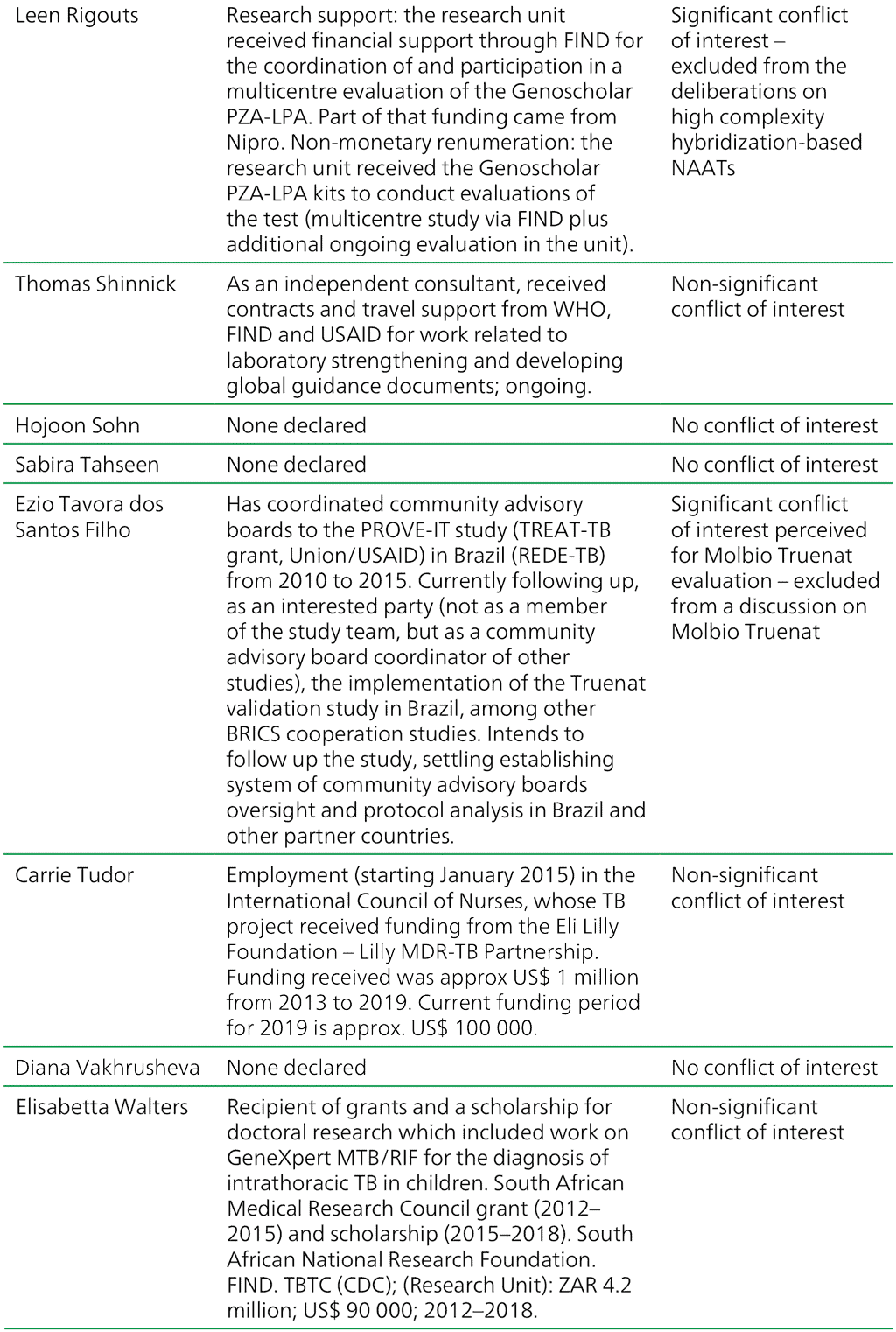
AIDS: acquired immunodeficiency syndrome; BRICS: Brazil, Russia, India, China and South Africa; CDC: Centers for Disease Control and Prevention; DNA: deoxyribonucleic acid; EDCTP: European and Developing Countries Clinical Trials Partnership; FIND: Foundation for Innovative New Diagnostics; GDG: Guideline Development Group; HIV: human immunodeficiency virus; IUATLD: International Union Against Tuberculosis and Lung Disease; MDR-TB: multidrug-resistant TB; NAAT: nucleic acid amplification test; NIH: National Institutes of Health; PEPFAR: United States President’s Emergency Plan for AIDS Relief; STI: sexually transmitted infection; TB: tuberculosis; TBTC: Tuberculosis Trials Consortium; USAID: United States Agency for International Development; WHO: World Health Organization; XDR: extensively drug-resistant.
Table A.2.2. Summary of the declarations of interest statements for the ERG members: “Molecular assays intended as initial tests”, 7–18 December 2020
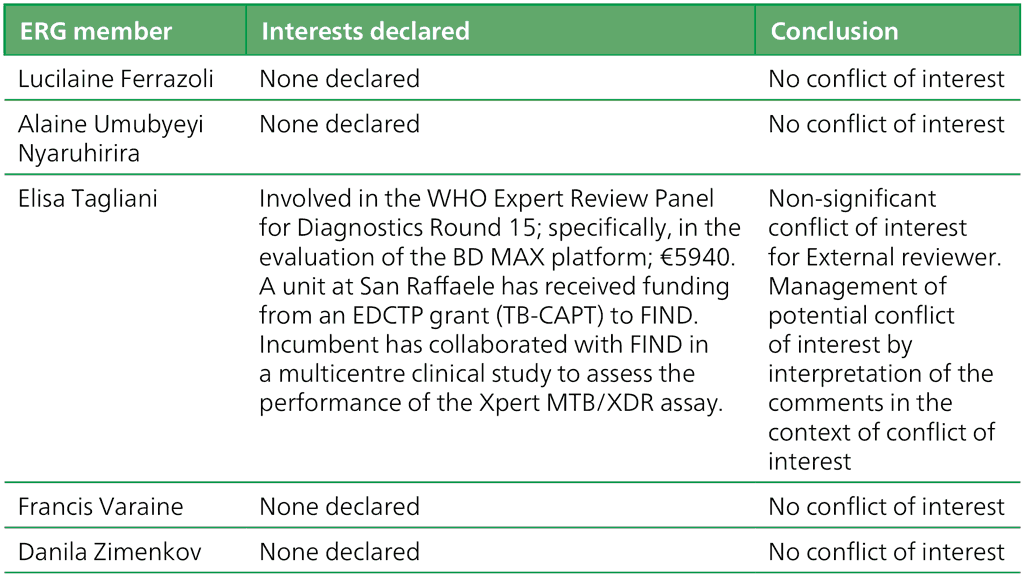
EDCTP: European and Developing Countries Clinical Trials Partnership; ERG: External Review Group; FIND: Foundation for Innovative New Diagnostics; WHO: World Health Organization.
Table A.2.3. Summary of the declarations of interest statements for the GDG: “Targeted next-generation sequencing” 2–5 May 2023
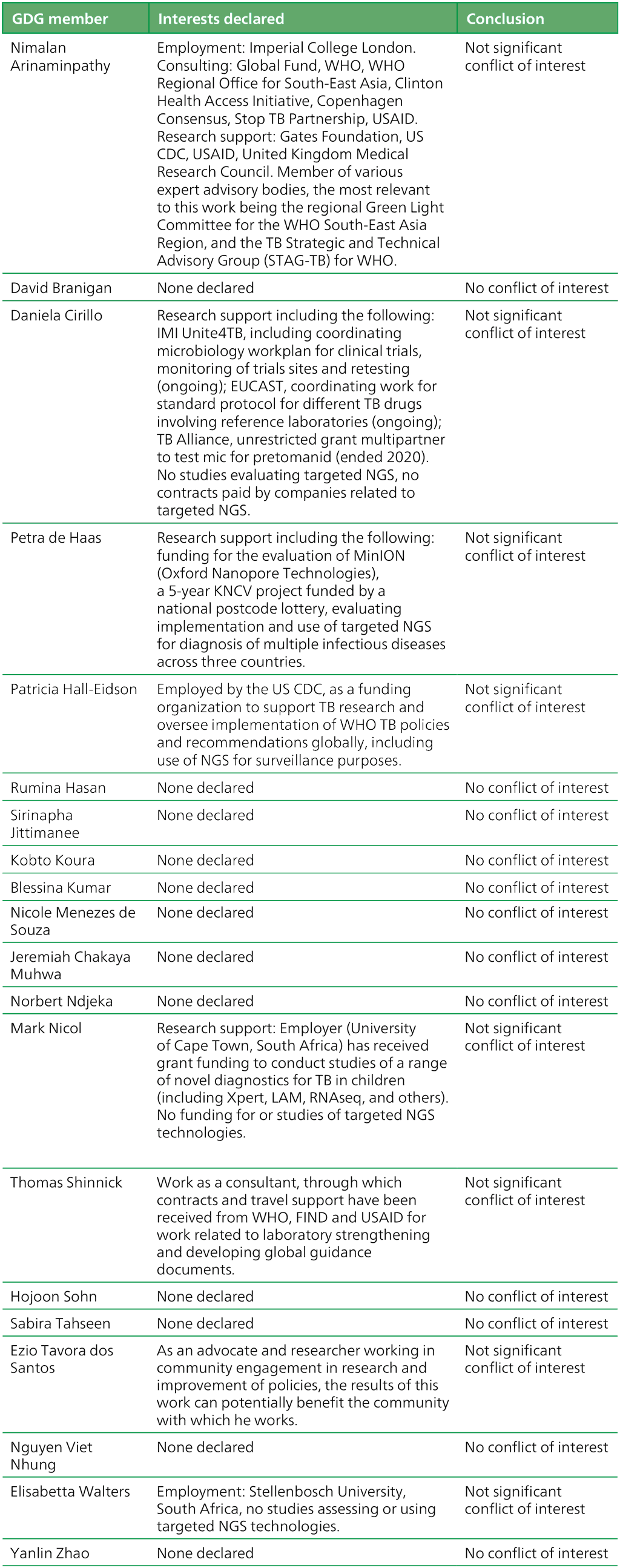
EUCAST: European Committee on Antimicrobial Susceptibility Testing; FIND: Foundation for Innovative New Diagnostics; GDG: Guideline Development Group; Global Fund: Global Fund to Fight AIDS, Tuberculosis and Malaria; IMI: Innovative Medicines Initiative; LAM: lipoarabinomannan antigen test; NGS: next-generation sequencing; TB: tuberculosis; United Kingdom: United Kingdom of Great Britain and Northern Ireland; USAID: United States Agency for International Development; US CDC: United States Centers for Disease Control and Prevention; WHO: World Health Organization.
Table A.2.4. Summary of the declarations of interest statements for the ERG: “Targeted Next-Generation Sequencing” 2–5 May 2023
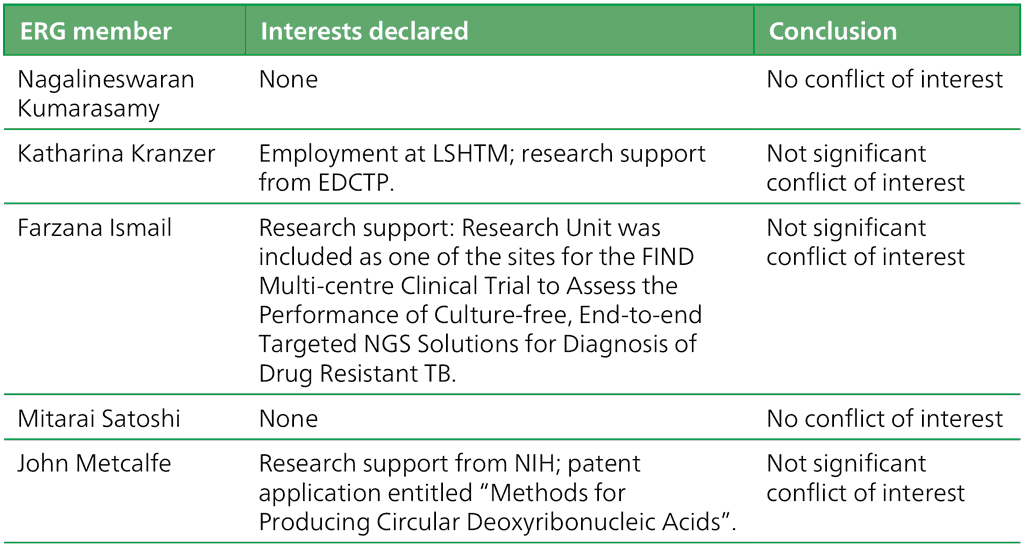
EDCTP: European and Developing Countries Clinical Trials Partnership; ERG: External Review Group; FIND: Foundation for Innovative New Diagnostics; LSHTM: London School of Hygiene & Tropical Medicine; NGS: next-generation sequencing; NIH: United States National Institutes of Health; TB: tuberculosis.
Table A.2.4. Summary of the declarations of interest statements for the GDG: “Low complexity nucleic acid amplification testing for detection of TB and resistance to rifampicin” 6–10 May 2024NEW
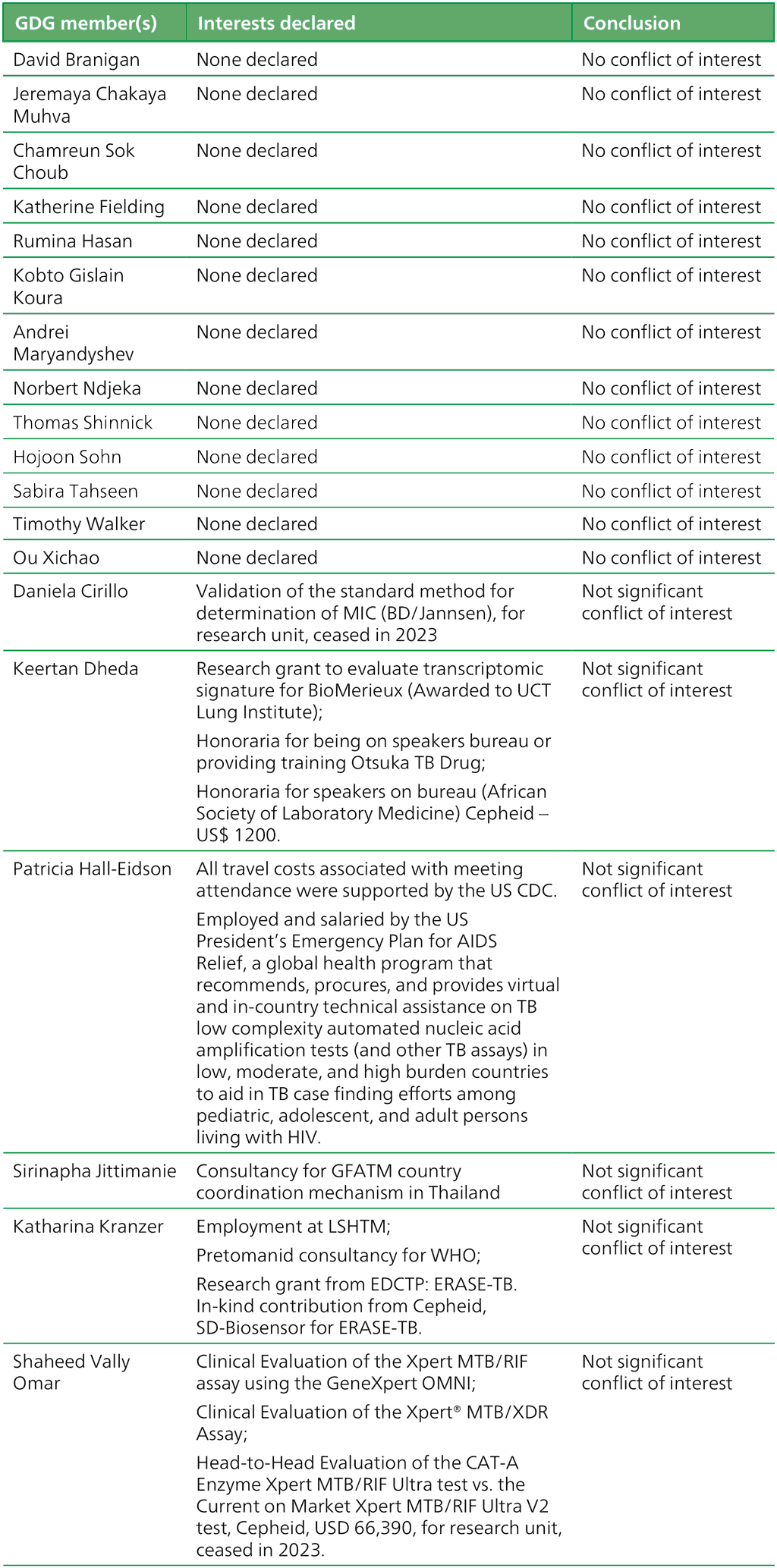
References for Annex 2
- Declaration of interests [website]. Geneva: World Health Organization; 2023 (https://www.who.int/about/ethics/declarations-of-interest).
- Handbook for guideline development 2nd ed. Geneva: World Health Organization; 2014 (https://apps.who.int/iris/handle/10665/145714).

 Feedback
Feedback