Book traversal links for 1266
Testing for TB infection increases the certainty that individuals targeted for treatment will benefit from it. However, there is no gold-standard test to diagnose TB infection. Both currently available tests – the TST and IGRAs – are indirect and require a competent immune response to identify people infected with TB. A positive test result by either method is not by itself a reliable indicator of the risk of progression to active disease. This section discusses the evidence and the recommendations for TB infection testing.
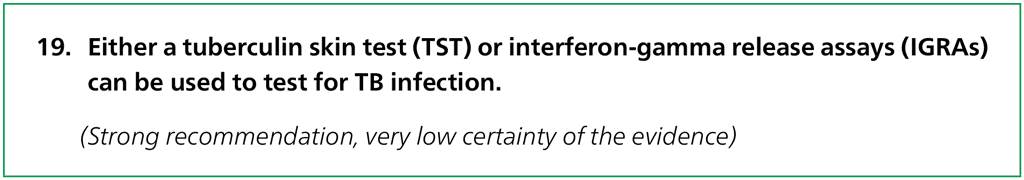
Recommendation
Justification
A systematic review has informed the comparison of the predictive performance of IGRAs and the TST for identifying incident active TB in countries with a TB incidence of more than 100 per 100 000 population (12). Only studies in which the TST was compared with IGRAs in the same population (i.e. “head-to-head” studies) were included. Relative risk ratios for TB for people who tested positive and those who tested negative with the TST and IGRAs were estimated.
Five prospective cohort studies were identified, with a total of 7769 participants. The pooled risk ratio estimate for the TST was 1.49 (95% CI: 0.79–2.80), and for IGRAs was 2.03 (95% CI: 1.18–3.50). Although the estimate for IGRAs was slightly higher than that for the TST, the 95% CIs for the estimates for the TST and IGRAs overlapped and were imprecise.
The GDG concluded that the comparison of the TST and IGRAs in the same population does not provide strong evidence that one test should be preferred over the other for predicting progression to active TB disease. The TST may require significantly fewer resources than IGRAs and may be more familiar to practitioners in resource-limited settings; however, recurrent global shortages and stock-outs of the TST reduce prospects for the scale-up of this test and for the programmatic management of TPT. The GDG also noted that equity and access could affect the choice and type of test used. The preferences of people to be tested and programmes depend on several factors, such as the requirement for an adequately equipped laboratory (e.g. for IGRAs) and possible additional costs for people being tested (e.g. for travel) and programmes (e.g. for infrastructure and testing). The GDG strongly recommended the two tests as equivalent options, with relatively similar advantages and disadvantages. The GDG stressed that the global shortage of the TST should be addressed urgently, and called for more investment into research on novel tests for TB infection with better predictive value. The GDG cautioned that imperfect performance of these tests can lead to false negative results, particularly in young children and immunocompromised individuals such as PLHIV with low CD4 counts. The GDG noted the importance of the tests to identify recent conversion from negative to positive, particularly among contacts of people with pulmonary TB, which is good practice when initiating TPT. Nevertheless, recent studies among health care workers in the USA tested serially for TB infection showed that conversions from negative to positive and reversions from positive to negative are more commonly identified with IGRAs than with the TST (13). Thus, clinical judgement must still be used to interpret the results of serial TB infection tests.
The evidence reviewed and the recommendations given apply only to the use of the two commercially available IGRAs (QuantiFERON-TB Gold In-Tube and T-Spot.
Evidence base
PICO question
Could IGRA be used as an alternative to the TST, to identify individuals most at risk of progression from TB infection to active TB in high TB incidence settings?
Evidence on intervention effect
Five prospective cohort studies were identified, with a total of 7769 participants; four of the studies were newly identified. Three of the studies were conducted in South Africa and two in India (14–18). The studies included PLHIV, pregnant women, adolescents, health care workers and household contacts. The pooled risk ratio estimate for the TST was 1.49 (95% CI: 0.79– 2.80), and for IGRAs was 2.03 (95% CI: 1.18–3.50). Although the estimate for IGRAs was slightly higher than that for the TST, the 95% CIs for the estimates for the TST and IGRAs overlapped and were imprecise. Furthermore, there was limited evidence for the predictive utility of the tests in specific at-risk populations.
Cost–effectiveness
IGRA testing is more costly than the TST and requires appropriate laboratory services. TST testing is less costly and can be performed in the field, but it requires a cold chain, two health care visits and training in intradermal injection, reading and interpretation. The incremental cost–effectiveness of IGRAs and the TST appears to be influenced mainly by their accuracy.
User perspective
The preferences of people to be tested and programmes depend on several factors, such as the requirement for an adequately equipped laboratory (e.g. for IGRAs) and possible additional costs for people being tested (e.g. for travel) and programmes (e.g. for infrastructure and testing).
Implementation considerations
Where it is feasible, TB infection testing is desirable to identify individuals at highest risk for developing active TB. However, it is not required in PLHIV or in household contacts aged under 5 years. In HIV-negative household contacts aged 5 years and older, and in other risk groups, TB infection tests are recommended, but their unavailability should not be a barrier to treating people who are judged to be at higher risk. The GDG noted that the availability and affordability of the tests could determine which TB infection test is used. Other considerations include the structure of the health system, feasibility of implementation and infrastructure requirements.
Operational difficulties should be considered in deciding which test to use. For example, IGRAs requires phlebotomy, which can be difficult, particularly in young children; they also require laboratory infrastructure, technical expertise and expensive equipment, and their sensitivity is reduced in children aged under 2 years and PLHIV. However, only a single visit is required to do an IGRA test (although patients may have to make a second visit to receive the result). The TST requires a cold chain, two health care visits and training in intradermal injection, reading and interpretation. One other practical advantage of IGRAs over the TST is that IGRAs are notsusceptible to a “booster response”, which makes a two-step approach necessary for the TST in situations where reactivity to the TST has waned since infection.
BCG vaccination plays a decisive role in reducing the specificity of the TST, although the GDG noted that the impact of BCG vaccination on the specificity of the TST depends on the strain of vaccine used, the age at which the vaccine is given and the number of doses administered. When BCG is given at birth, as is the case in most parts of the world, it has a variable, limited impact on TST specificity (19).
The GDG agreed that a history of BCG vaccination has a limited effect on interpretation of TST results later in life; hence, BCG vaccination should not be a determining factor in selecting a test. Neither the TST nor IGRAs are to be used to diagnose active TB disease; also, they are not to be used for diagnostic work-up of adults suspected of having active TB.
Research priorities
There is a critical need for diagnostic tests with improved performance and predictive value for progression to active TB. In addition, the performance of TB infection tests should be evaluated in various risk groups, to assess reinfection and to understand how best to use available tools in each population (e.g. in combination, or sequential use of the TST and IGRAs).
Data synthesis was structured around the preset PICO question, as outlined above. See Web Annex H for additional information on evidence synthesis and analysis.

 Feedback
Feedback