6. Starting antiretroviral therapy in patients on MDR/RR-TB regimens
Recommendation 6.1 Starting ART

Justification and evidence
The recommendation in this section addresses one PICO question:
 TB KaSPar
TB KaSPar
 Feedback
Feedback
Recommendation 6.1 Starting ART

Justification and evidence
The recommendation in this section addresses one PICO question:
People who receive MDR/RR-TB regimens need to be monitored during treatment using relevant clinical and laboratory testing schedules. Response to treatment and toxicity are monitored through regular history taking, physical examination and CXR; special tests (e.g. audiometry, visual acuity tests, peripheral neurological examination and electrocardiography); and laboratory monitoring. Using smear microscopy or culture to assess the conversion of bacteriological status is an important way to assess treatment response.
Recommendations 4.1–4.2 Treatment of Hr-TB

Justification and evidence
The recommendations in this section address one PICO question:
Recommendations 3.1–3.17 Longer regimens
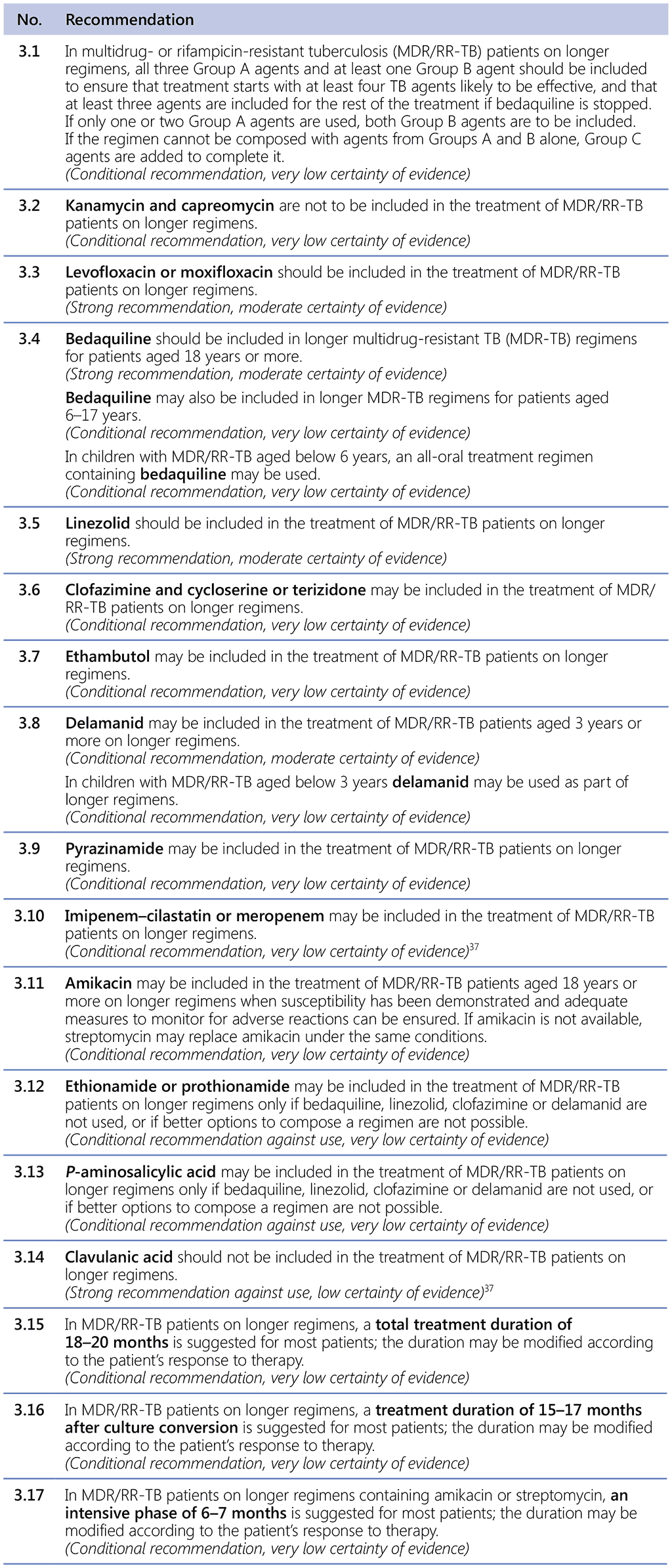
Table 3.1 gives details of the grouping of medicines recommended for use in longer MDR-TB regimens; the groups are summarized here for clarity:

Remarks
Remarks
These guidelines are primarily targeted at policy-makers in ministries of health, or managers of NTPs who formulate country-specific TB treatment guidelines or are involved in the planning of TB treatment programmes. It is expected that these updated recommendations will also be used by health professionals, including doctors, nurses and educators working in governmental and nongovernmental organizations, and by technical agencies involved in treating patients and organizing treatment services.
The recommendations for the treatment of DR-TB that are presented in this document have been derived from earlier WHO guideline documents (Box 1), and a WHO guideline development conducted in June 2024. These recommendations supersede the WHO consolidated guidelines on tuberculosis. Module 4: Treatment – drug-resistant tuberculosis treatment, that were published in 2022 (3).
This chapter provides specific recommendations on the treatment of DR-TB, including the use of regimens for rifampicin-susceptible, isoniazid-resistant TB (Hr-TB), shorter and longer regimens for MDR/RR-TB, monitoring patient response to MDR/RR-TB treatment, starting ART in patients on secondline anti-TB regimens, undertaking surgery for patients on DR-TB treatment and co-administration of MDR/RR-TB and HCV therapies.
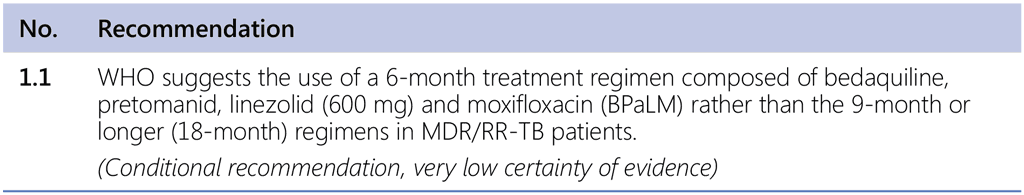
The 2024 GDG meeting convened by WHO resulted in two new recommendations: the use of a new 6-month regimen and modified 9-month regimens.