Book traversal links for 1266
This chapter provides specific recommendations on the treatment of DR-TB, including the use of regimens for rifampicin-susceptible, isoniazid-resistant TB (Hr-TB), shorter and longer regimens for MDR/RR-TB, monitoring patient response to MDR/RR-TB treatment, starting ART in patients on secondline anti-TB regimens, undertaking surgery for patients on DR-TB treatment and co-administration of MDR/RR-TB and HCV therapies.
The 2024 GDG meeting convened by WHO resulted in two new recommendations: the use of a new 6-month regimen and modified 9-month regimens.
Access to the new evidence was achieved through close collaboration and engagement with national TB programmes (NTPs), researchers, and not-for-profit organizations investigating the effectiveness and safety of these interventions.
The new evidence appraised during the 2024 GDG meeting included a new 6-month regimen based on bedaquiline (B), delamanid (D), and linezolid (L) in combination with either levofloxacin (Lfx) or clofazimine (C) or both (BEAT-TB clinical trial in South Africa, NCT04062201) and a group of modified 9-month regimens for the treatment of patients with MDR/RR-TB without fluoroquinolone resistance (endTB clinical trial, NCT02754765).
Table A describes the evidence that was generously shared by researchers and NTPs with WHO/GTB for DR-TB treatment guideline updates in in 2019, 2021 and 2024.
Table A. Evidence available for the guidelines updates
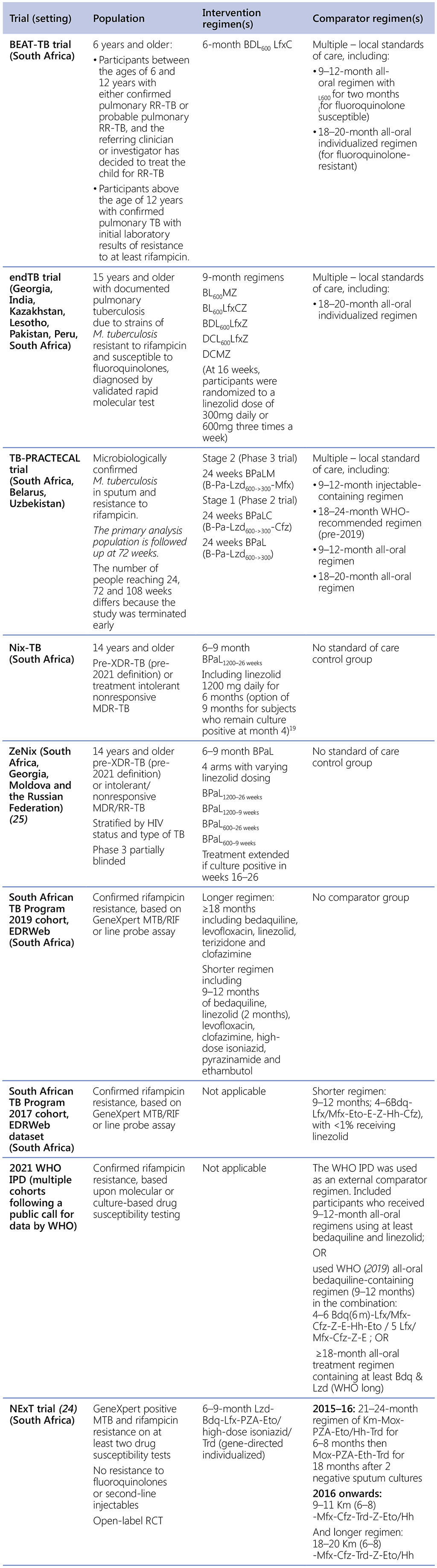
19 21 patients in the Nix-TB study received linezolid 600 mg per day, at the beginning of the recruitment period.
BPaL: bedaquiline, pretomanid and linezolid; BPaLC: bedaquiline, pretomanid, linezolid and clofazimine; BPaLM: bedaquiline, pretomanid, linezolid and moxifloxacin; BLMZ: bedaquiline, linezolid, moxifloxacin and pyrazinamide; BLLfxCZ: bedaquiline, linezolid, levofloxacin, clofazimine and pyrazinamide; BDLLfxZ: bedaquiline, delamanid, linezolid, levofloxacin and pyrazinamide; DCLLfxZ: delamanid, clofazimine, linezolid, levofloxacin and pyrazinamide; DCMZ: delamanid, clofazimine, moxifloxacin and pyrazinamide BDLLfxC: bedaquiline, delamanid, linezolid, levofloxacin and clofazimine; HIV: human immunodeficiency virus; IPD: individual patient dataset; M. tuberculosis; Mycobacterium tuberculosis; MDR-TB: multidrug-resistant TB; RCT: randomized controlled trial; TB: tuberculosis; WHO: World Health Organization; preXDR-TB: pre-extensively drug-resistant TB.

 Feedback
Feedback