Book traversal links for WHO TB KNOWLEDGE SHARING PLATFORM
People who receive MDR/RR-TB regimens need to be monitored during treatment using relevant clinical and laboratory testing schedules. Response to treatment and toxicity are monitored through regular history taking, physical examination and CXR; special tests (e.g. audiometry, visual acuity tests, peripheral neurological examination and electrocardiography); and laboratory monitoring. Using smear microscopy or culture to assess the conversion of bacteriological status is an important way to assess treatment response.
NTPs should aim for complete registration of patients with MDR/RR-TB through follow-up and monitoring of treatment outcomes as part of national surveillance. Regular review of MDR/RR-TB cohort data is essential. The prospective collection of accurate data for key variables at the case-based level, using an electronic register, is strongly advised, both for the benefit of the individual patient, and to inform revisions of local and global policies.
The WHO framework for aDSM needs to be applied to patients on any type of MDR/RR-TB regimen, to ensure appropriate action and an acceptable level of monitoring for AEs and prompt response to such events, alongside monitoring for treatment outcomes. The rationale for aDSM is largely supported by the increasing use worldwide of combinations of new and repurposed medications in MDR/RR-TB treatment regimens. Additional evidence generated on AEs will be important to build the evidence base on the safety of the new regimens in varied settings.
Access to reliable DST for bedaquiline, linezolid and all medicines composing the regimen is essential to investigate reasons for lack of bacteriological and clinical improvement – in an ideal situation, the DST for all second-line medicines used in regimens would be available. This should not, however, delay the initiation of life-saving treatment. Before starting a 9-month regimen, the patient’s bacteriological status should be available, with confirmation of TB disease, MDR/RR-TB (as a minimum) and FQ susceptibility. Country programmes need to strengthen and increase access to FQ DST and undertake surveillance for emerging drug resistance, including for bedaquiline and all second-line medicines for which reliable DST is available. The FQ DST is also important to support the prescription of the relevant combination of 6-month regimens – BPaLM, BPaL, BDLLfxC, BDLLfx or BDLC – to maximize efficacy and prevent unnecessary potential toxicity. The need for FQ DST should not be a barrier to starting treatment with 6-month regimens, because each regimen has a combination that can be started even though the FQ DST is not yet available.
Country programmes are also strongly encouraged to establish the DST capacity to test for resistance to bedaquiline and linezolid at baseline (particularly in cases demonstrating FQ resistance), and to test samples from patients with no bacteriological conversion after 4 months or recurrences while on the 6-month regimens. The implementation of 9-month regimens requires the use of routine DST to FQ, not only for patient selection but also to monitor the acquisition of resistance (collection of strains for sequencing should be considered). Critical accompaniments to the treatment recommendations in these guidelines are access to DST for medicines for which there are reliable methods and the development of other methods for newer medicines (e.g. sequencing).
ECG may be indicated because in the future, more regimens may have two or three agents that are expected to prolong the QT interval. When QTc prolongation is identified, it is adviseable to check whether the serum potassium, calcium and magnesium are abnormal (and correct if necessary). Treating clinicians are also advised to obtain an ECG before initiation of treatment.
Audiometry and specific biochemical tests should also be made available whenever certain agents are included in the regimens. Treatment in pregnancy with postpartum surveillance for congenital anomalies will help to inform future recommendations for MDR/RR-TB treatment during pregnancy.
It is good practice to assess patients for symptoms and signs of liver disease (e.g. fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness and hepatomegaly); and to conduct laboratory tests such as ALT, AST, alkaline phosphatase and bilirubin. More frequent monitoring of indicators of hepatic toxicity is strongly advised for all MDR/RR-TB regimens.
Monitoring changes in dosing and duration of linezolid, in particular (when needed), will be important, to inform the future evidence base on the wider use of the 6-month or 9-month regimens, and the tolerability of linezolid in these regimens.
Treatment administration coupled with patient support can boost adherence; it can also ensure optimal drug effectiveness and safety of patients on treatment. Measures to support patient adherence (e.g. facilitating patient visits to health care facilities or home visits by health care staff, or using digital technologies for daily communication) may be important to retain patients on treatment, even when a regimen is comparatively short. WHO recommendations on care and support are given in Chapter 3.
A CXR at baseline and the end of treatment can help in judging the treatment response, which should be monitored by monthly sputum smear microscopy and culture (ideally at the same frequency). Failure to convert sputum culture at or after the fourth month of treatment or recurrence is a potential predictor of a failing treatment regimen. Persistence of culture positivity beyond that point should trigger DST and a potential review of the regimen. Where feasible, it is also important to follow up patients 12 months after the completion of treatment for possible relapse, including with sputum culture and smear. In the 2018 update of the guidelines, a separate recommendation was made on the use of culture and microscopy to monitor bacteriological response during treatment. A suggested monitoring schedule is provided in the WHO consolidated operational handbook on tuberculosis. Module 4: Treatment and care (69).
Recommendation 5.1 Monitoring patient response
Justification and evidence
The recommendation in this section addresses the following PICO question:
PICO question (MDR/RR-TB, 2018). In patients with MDR/RR-TB treated with longer or shorter regimens composed in accordance with WHO guidelines, is monitoring using monthly cultures, in addition to smear microscopy, more likely to detect non-response to treatment?
Previous studies have indicated that monthly culture is the optimum strategy to detect non-response as early as possible and was conditionally recommended by WHO in 2011 as the preferred approach (13, 118, 119). The findings of the evidence review and analysis performed for this question are expected to influence the continued validity, in its present form, of the 2011 WHO recommendation (13). Since then, significant changes in MDR-TB treatment practices have taken place on a large scale globally, such as the wider use of later-generation fluoroquinolones, bedaquiline and linezolid; a tendency towards an intensive phase of longer duration; and the widespread use of the shorter regimen, which could influence the speed and durability of culture conversion during the continuation phase, when this PICO question is of greatest relevance.
Achieving sustained bacteriological conversion from positive to negative is widely used to assess response to treatment in both drug-susceptible TB and DR-TB. Culture is a more sensitive test for bacteriological confirmation of TB than direct microscopy of sputum and other biological specimens. Culture also facilitates phenotypic testing for DST, a critical consideration in TB diagnostics. However, performing culture requires considerable logistical organization and a well-equipped laboratory to limit cross-contamination, ensure proper bacterial growth and match other quality standards. Apart from the resource requirements, culture results become available after a significant delay of weeks or months, contrasting markedly with the relative immediacy of the result of direct microscopy (although microscopy cannot confirm mycobacterial viability). Although molecular techniques can now provide a rapid and reliable diagnosis, they cannot replace culture or microscopy for the monitoring of bacteriological status during treatment.
The evidence used to explore the added value of culture over sputum smear microscopy alone, and the optimal frequency of monitoring, was obtained from a subset of the IPD reported to WHO by South Africa for the 2018 update. These observational data from South Africa comprised 26 522 patients overall. Of these, 22 760 records were excluded from the dataset for the following reasons: 11 236 had a treatment outcome of death or LTFU; 698 had a successful treatment outcome but had received less than 17.5 months of treatment; 1357 had fewer than six culture samples recorded; 1632 had no baseline culture recorded; 2502 were baseline culture negative; 2920 were smear negative at baseline or had a missing smear at baseline; and 2415 had insufficient smear data to match the culture data. This left 3762 MDR/RR-TB patients (with 1.8% being children; i.e. aged <15 years) treated with longer MDR-TB regimens between 2010 and 2015, who had both monthly smear and culture data throughout treatment to address PICO question 11 (MDR/RR-TB, 2018). About 60% of these patients were PLHIV. The analysis focused on whether monthly culture versus monthly smear microscopy or culture every 2 months is needed to not miss treatment failure in MDR/RR-TB patients on treatment. The odds of treatment failure in patients who do not convert at 6 months or later was also discussed (see Implementation considerations and Table 5.1). The data could not address the outcome on acquisition (amplification) of additional drug resistance, nor could it assess directly whether the frequency of culture or smear microscopy had an identical effect on failure in patients on the 9–12-month shorter MDR-TB regimen, as envisaged in the original PICO question 11 (MDR/RR-TB, 2018). Based on an assessment of the certainty of the evidence, carried out using predefined criteria and documented in GRADEpro, the test accuracy certainty of the evidence was rated as moderate.
The IPD meta-analysis compared the performance of the two methods in terms of sensitivity and specificity, and culture testing once a month compared with once every 2 months (to assess the minimum frequency of testing needed to not unnecessarily delay any revision of the treatment). The focus of the analysis was to compare how the two tests performed in terms of predicting treatment failure or relapse.
The main findings of the analysis were that monthly culture had a higher sensitivity than monthly smear microscopy (0.93 vs 0.51) but slightly lower specificity (0.97 vs 0.99). Likewise, the sensitivity of culture done every month was much higher than once every 2 months (0.93 vs 0.73) but had a slightly lower specificity (0.97 vs 0.98). Monthly culture increased the number of patients detected with a true positive bacteriological result by 13 per 1000 patients and reduced false negative results by 13 per 1000 patients when compared with sputum smear microscopy alone. In contrast, monthly culture was estimated to lead to 17 per 1000 fewer true negative results and 17 per 1000 more false positive results for treatment failure, implying that treatment may be prolonged in the case of false positivity or missed true negativity. The added inconvenience to the patient and programme is considered relatively small, given that taking sputum and many other biological specimens is usually non-invasive and routine practice in many programmes. In a setting where testing is repeated at monthly intervals, a single false positive test result is unlikely to prove harmful to the patient because treatment decisions usually rely upon at least two consecutive positive results (to denote prolonged positivity or reversion) and the effect of one spurious result would last only until the test repeated 1 month later is reported.
The crude odds of treatment failure increased steadily with each additional month without bacteriological conversion, from 3.6 at the end of the first month to 45 at the eighth month when using culture (Table 5.1). However, no discrete cut-off point (to serve as a reliable marker of a failing regimen) could be discerned at which the odds of failure increased sharply when monitoring with either sputum smear microscopy or culture. The threshold for when to change treatment thus depends on the clinician’s desire to minimize the risk of failure and, in particular, to limit the risk of prolonging a failing regimen.
Table 5.1. Crude odds ratios (95% CLs) of treatment failure in MDR/RR-TB patients without sputum conversion by the end of successive months of treatment compared with patients who converted, by testing method used; IPD meta-analysis for PICO question 7 MDR/RR-TB, 2018 (South Africa, n=3762)
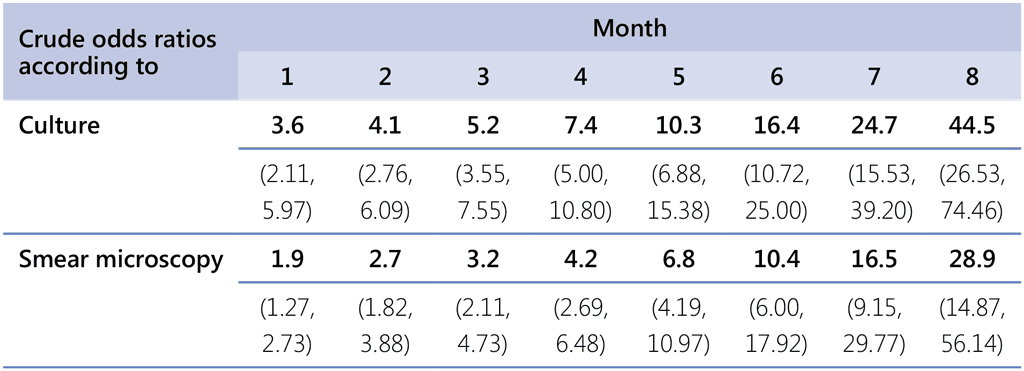
CL: confidence limits; IPD: individual patient data; MDR/RR-TB: multidrug-resistant or rifampicin-resistant tuberculosis; PICO: population, intervention, comparator and outcome.
There was moderate certainty in the estimates of test accuracy and the GDG considered that, under normal conditions, culture would always be a more sensitive test of positive bacterial status than sputum smear microscopy. However, the overall quality of the evidence was judged to be low. The effects observed may vary in patients or populations with a profile markedly different from the one included in the analysis, such as low HIV-prevalence settings, children, patients with extrapulmonary forms of disease or those treated with the shorter MDR-TB regimen. The 3762 patients included in the analysis had similar clinical characteristics to the 22 760 individuals excluded, although they were slightly less likely to be HIV coinfected, have a history of previous treatment or have second-line drug resistance. Conversely, the rate of failure in those included in the analysis was only 3% compared with 12.7% of those excluded from the analysis.
Subgroup considerations
The recommendation would apply to any longer regimen, regardless of the number of Group A, B or C agents used and whether an injectable (intensive) phase was used or not. The GDG considered that the findings may apply to other key patient subgroups.
Patients aged below 15 years with MDR/RR-TB
Patients aged below 15 years with MDR/RR-TB comprised less than 2% of the IPD meta-analysis analysed for PICO question 11 (MDR/RR-TB, 2018). Younger children usually cannot produce sufficient sputum spontaneously to allow a bacteriological diagnosis (many are typically sputum smear microscopy negative). In these patients, culture may be a more sensitive way to detect viable TB bacilli even if very few organisms are present in the sputum or other samples that are below the detection threshold of direct microscopy. However, in children who are unable to expectorate, gastric aspirates or induced sputa may be possible, but the repetition of such tests at monthly frequency may not be acceptable.
Extrapulmonary disease
Extrapulmonary disease is commonly paucibacillary; therefore, biological specimens may contain few or no bacilli. In such situations, detection of persistent disease is more likely with culture, although collection of samples often poses problems. Direct microscopy should still be attempted because it may determine positivity much faster than culture.
HIV-negative individuals
HIV-negative individuals with TB typically have higher bacterial counts in the sputum and a greater likelihood of detection with smear microscopy. In such a situation, it might be expected that the difference in test sensitivity between smear and culture would be less extreme, because fewer patients would have subthreshold bacterial counts. However, past studies on datasets from multiple sites in which HIV positivity was low reported findings that led to the WHO recommendation, even in 2011, for joint use of both microscopy and culture, preferably every month.
Patients on the shorter MDR-TB regimen
Patients on the shorter MDR-TB regimen have a much shorter duration of intensive phase and total treatment. They receive seven drugs in the initial phase and, if fully compliant with the inclusion and exclusion criteria, usually have a more favourable prognostic outlook than other MDR-TB patients. Programmes may thus consider that patients on a shorter MDR-TB regimen may need less frequent or no culture to monitor treatment. Although the current analysis did not include patients treated with shorter regimens, the GDG proposes that programmes that implement this regimen should aim for more frequent culture testing, especially after the intensive phase, to confirm bacteriological cure in patients who complete treatment without signs of failure. Any sign of recurrence after termination of treatment should also be investigated using sputum smear microscopy, culture and DST.
Implementation considerations
Good-quality sputum specimens are necessary to ensure that laboratories can diagnose TB properly. In addition, laboratories should have sufficient space to ensure the quality, safety and efficiency of the services provided to clients whose samples are tested, and to ensure the safety of laboratory personnel, patients and visitors (120). Some countries experience difficulties with the implementation and quality assurance of sputum culture, which affects this recommendation because it is dependent on access to quality-assured laboratories that can offer TB culture. Sputum smear and culture examinations are also dependent on the quality of the sputum produced, so care should be taken to obtain adequate specimens and transport them to the laboratory according to standard procedures, to maintain the viability of the bacilli and thus obtain a valid culture result.
In programmatic settings, the practitioner treating MDR-TB patients is typically guided not only by bacteriological tests but also by markers of response to treatment or of disease progression, such as the patient’s general condition, weight gain over time, resolution of disease manifestations, blood indices and results of imaging (e.g. chest radiography). The potential use of Xpert MTB/RIF assay in monitoring treatment response has yet to be determined (121, 122).
The implementation of more frequent microbiological testing would require appropriate resources to be made available, both for the laboratories undertaking the tests and for the patient, who may have to spend more time visiting the facilities and, at times, pay for the testing. Patient values and preferences need to be considered to ensure a more acceptable service and patient-centred delivery of care. Increased monitoring should not be done at the expense of overburdening the laboratory services or upsetting health equity by displacing resources from other essential components of the programme.
Monitoring and evaluation
Culture and microscopy results for tests performed in patients on MDR-TB treatment should be captured in the second-line TB treatment register as well as the respective laboratory registers (94). Sometimes these registers may exist as part of an electronic laboratory or patient information system, which makes it much easier for multiple users to access the data in real time and can also help to limit errors. It is important for the programme manager to assess the records in the second-line TB treatment register for completeness of testing using both culture and sputum smear microscopy, any discordance between the two modalities, and whether decisions on regimen changes or assignment of outcome are coherent (e.g. does a case have sufficient negative culture test results available to be classified as “cured”?). Performance indicators help to improve the quality of care; such indicators include contamination rates, turnaround times and proportion of culture tests done without results being recorded in the patient information system. In the case of repeated positive cultures, repeat testing for drug susceptibility or resistance is important.

 Feedback
Feedback