Book traversal links for 1266
Recommendations 3.1–3.17 Longer regimens
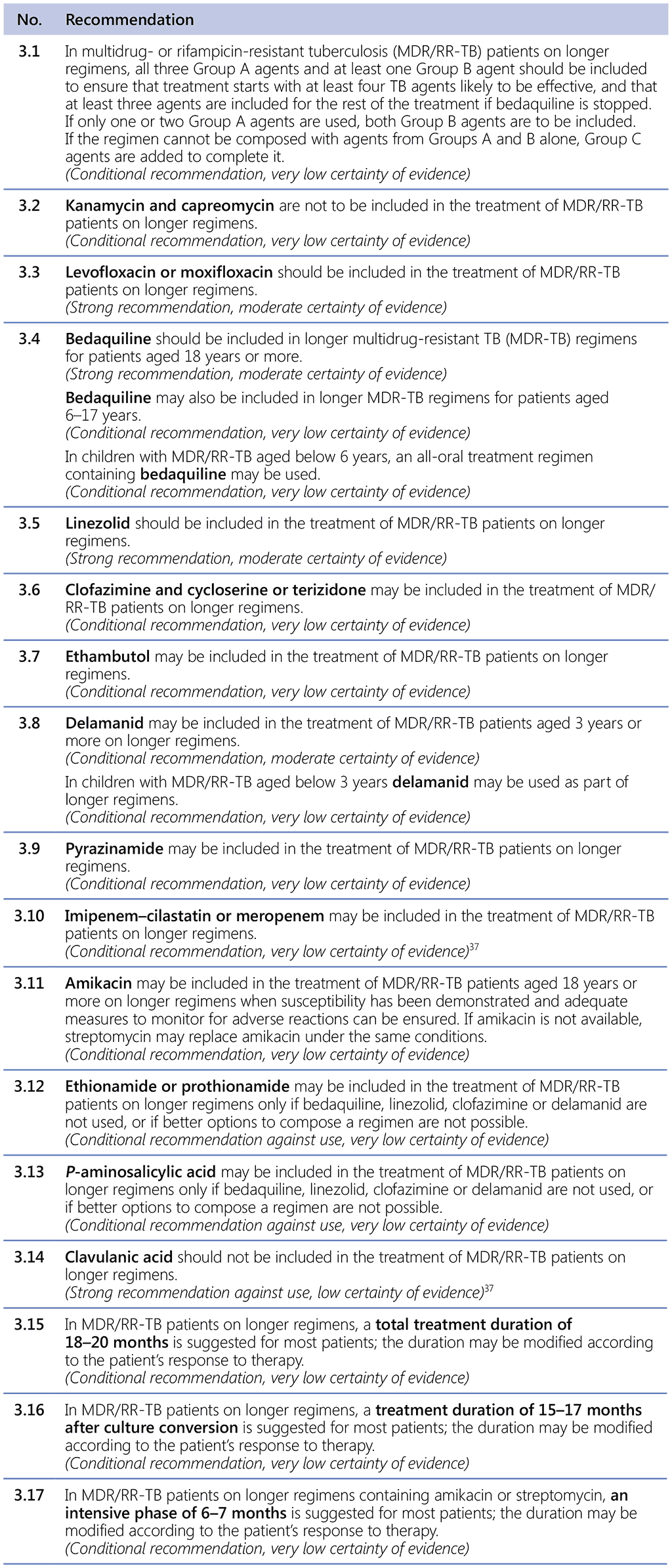
Table 3.1 gives details of the grouping of medicines recommended for use in longer MDR-TB regimens; the groups are summarized here for clarity:
- Group A = levofloxacin or moxifloxacin, bedaquiline and linezolid;
- Group B = clofazimine, and cycloserine or terizidone; and
- Group C = ethambutol, delamanid, pyrazinamide, imipenem–cilastatin or meropenem, amikacin (or streptomycin), ethionamide or prothionamide, and p-aminosalicylic acid.
Justification and evidence
This section refers to recommendations on MDR/RR-TB treatment regimens that are of longer duration than the regimens described in the previous sections.
PICO questions
The recommendations in this section address PICO questions formulated in 2018 and 2019. The questions formulated in 2018 were as follows:
PICO question 3–2018 (MDR/RR-TB, 2018): In patients with MDR/RR-TB, which individual agents are more likely to improve outcomes when forming part of a longer regimen conforming to WHO guidelines?³⁸
PICO question 4–2018 (MDR/RR-TB, 2018): In patients with MDR/RR-TB on longer regimens composed in accordance with WHO guidelines, are outcomes safely improved with fewer or more than five effective medicines in the intensive phase?
PICO question 5–2018 (MDR/RR-TB, 2018): In patients with MDR/RR-TB on longer regimens composed in accordance with WHO guidelines, are outcomes safely improved with an intensive phase shorter or longer than 8 months?
PICO question 6–2018 (MDR/RR-TB, 2018): In patients with MDR/RR-TB on longer regimens composed in accordance with WHO guidelines, are outcomes safely improved with a total duration shorter or longer than 20 months?
PICO question 7–2018 (MDR/RR-TB, 2018): In patients with MDR/RR-TB on longer regimens composed in accordance with WHO guidelines, what is the minimum duration of treatment after culture conversion that is most likely to improve outcomes?
The two relevant PICO questions considered by the GDG for the 2020 update were as follows:
PICO question 8–2019 (MDR/RR-TB, 2019): In MDR/RR-TB patients, does a treatment regimen containing bedaquiline for more than 6 months safely improve outcomes when compared with bedaquiline for up to 6 months as part of longer regimens otherwise conforming to WHO guidelines?
PICO question 9–2019 (MDR/RR-TB, 2019): In MDR/RR-TB patients, does concurrent use of bedaquiline and delamanid safely improve outcomes when compared with other treatment regimen options otherwise conforming to WHO guidelines?
Two additional PICO questions were reviewed in 2021 as part of the GDG formed to update childhood TB guidelines (30):
PICO question 1–2021 (Childhood TB, 2021): In MDR/RR-TB patients aged below 6 years, should an all-oral treatment regimen containing bedaquiline versus other regimens conforming to WHO guidelines without bedaquiline be used?
PICO question 2–2021 (Childhood TB, 2021): In MDR/RR-TB patients aged below 3 years, should an all-oral treatment regimen containing delamanid versus other regimens conforming to WHO guidelines without delamanid be used?
Recommendations for the design of longer MDR-TB regimens have been issued by WHO for several years and have been implemented in many countries worldwide (2, 6, 13, 16). The recommendations in this section cover all forms of MDR/RR-TB; they include treatment of patients with strains resistant to rifampicin and susceptible to isoniazid (i.e. RR-TB), or with additional resistance to isoniazid (i.e. MDR-TB), or with resistance to other medicines (i.e. pre-XDR or XDR-TB). All patients with TB – children or adults – diagnosed with strains shown to be resistant to rifampicin can be placed on an MDR/RR-TB treatment regimen (2).
The likelihood of treatment success in MDR/RR-TB patients on longer regimens depends on factors related to the patient and strain of TB (e.g. severity of disease, resistance patterns and comorbidities), and access to health care (e.g. regimens with sufficient effective agents, medications of good quality, management of AEs and patient support). Longer regimens with sufficient effective agents are known to increase the likelihood of cure and lower the risk of death in adults and children (70–73). The composition of longer regimens is governed by the selection of individual medicines considered to be effective, and by a need to combine sufficient medicines to maximize the likelihood of relapse-free cure without increasing toxicity. Regimens may be of standardized (fixed) composition or may be individualized to the patient’s needs. Longer regimens usually last 18–20 months or more; this document provides recommendations on the duration of such regimens, updated since publication of the 2011 WHO guidelines (13). In summary, in 2018, a total treatment duration of 18–20 months and a treatment duration of 15–17 months after culture conversion were suggested for most patients, with the duration being modified according to the patient’s response to therapy.
Evidence base and analyses
Ahead of the GDG discussion in 2018, WHO made a public call for individual MDR/RR-TB patient data, complete with results of treatment (74). IPD meta-analysis in adults and children treated with longer MDR/RR-TB regimens allows the study of useful correlates of outcome, including the regimen composition (70–72). The evidence base for the effectiveness of many of the medicines used in MDR/RR-TB regimens relies on the 2018 IPD meta-analysis. In turn, this IPD meta-analysis relies heavily on observational studies, only a few of which have employed randomized controlled designs (21); hence, the overall certainty of evidence is often graded as low or very low. The sources of data used by the GDGs to address the PICO questions in this section are summarized below.
PICO question 3–2018 (MDR/RR-TB, 2018) (choice of individual medicines)
First, to analyse treatment success, treatment failure, relapse and death for the individual medicines in longer regimens, the 2018 IPD meta-analysis was used, with 13 104 records from 53 studies in 40 countries. The 2018 IPD contained datasets from preceding years and from several countries, including a large dataset from South Africa with many patients treated with bedaquiline-containing regimens. Second, to analyse AEs resulting in permanent discontinuation of individual medicines in longer regimens, a subset of 5450 records from 17 studies in the 2018 IPD was used, supplemented with additional information from 10 other studies that only reported AEs for either bedaquiline (n=130), linezolid (n=508) or carbapenems (n=139).
In addition to these data, the GDG 2018 assessed unpublished results from the Phase 3 Trial 213 of delamanid (75, 76) and safety and pharmacological exposure data from unpublished paediatric studies of bedaquiline (Phase 2 TMC207-C211 and Phase 1–2 IMPAACT P1108) and delamanid (Phase 1 242–12–245, Phase 1 242–12–232, Phase 2 242–07–204 and Phase 2 242–12–233). The GDG 2018 also searched the literature for studies reporting outcomes of patients treated with agents other than those included in the 2016 guidelines (e.g. perchlozone, interferon gamma and sutezolid).
PICO question 4–2018 (MDR/RR-TB, 2018) (number of agents likely to be effective)
To analyse treatment success, treatment failure, relapse and death for the optimal number of medicines to be included in longer regimens, the data were derived from a subset of 8957 patients in 47 studies included in the IPD used for PICO question 2–2018 (MDR/RR-TB, 2018) above. Of these, 3570 patients in 16 studies had information on the start and end dates for individual medicines in which DST was reported, and 5387 patients in 31 studies had information on individual medicines used in both the intensive and continuation phases of treatment, as well as DST results. This question focused on the number of agents in the intensive phase; hence, patients who did not receive an injectable agent or in whom an initial intensive phase was not defined were excluded (n=476). Patients who were designated “cured” or “treatment completed” but received less than 18 months of treatment – the minimum duration for longer regimens recommended by WHO in the past – were also excluded (n=346). In cases where DST results were available, a medicine was considered effective if results showed susceptibility, and was considered not effective if results showed resistance. Where DST results were missing, two situations existed. First, if the prevalence of resistance to that medicine was less than 10% in the same population (i.e. from the same country or study site if within one country, or overall at all sites if local data were not available), then the medicine was counted as effective. This situation applied to the following agents: cycloserine or terizidone, linezolid, clofazimine, bedaquiline, the carbapenems and delamanid. Second, if the prevalence of resistance to that medicine was more than or equal to 10% in the same population (from the same country or study site if within one country, or overall, at all sites if local data were not available), then imputed DST results were used to determine effectiveness if DST was missing. If the imputed DST result showed susceptibility, then the medicine was counted as effective; if the imputed DST result showed resistance, then the medicine was not counted as effective. This situation applied to the following agents: pyrazinamide, ethambutol, secondline injectable agents, fluoroquinolones, p-aminosalicylic acid, ethionamide and prothionamide. The following agents were not included when counting the number of medicines likely to be effective (regardless of any DST result that may have been available): isoniazid (including high-dose isoniazid), rifampicin, rifabutin, thioacetazone, amoxicillin–clavulanate and macrolide antibiotics.
Subsets of the main 2018 IPD meta-analysis with 13 104 patients overall from 53 studies in 40 countries were analysed for the risk of treatment failure and relapse versus success associated with different durations in these three recommendations on the duration of treatment (see Annex 5 for the GRADE tables, and Annex 6 for the analysis plan). Patients were followed up for relapse but numbers of patients reported with relapse were relatively small. The three IPD subsets for PICO questions 5, 6 and 7–2018 are discussed below.
PICO question 5–2018 (MDR/RR-TB, 2018) (different durations of the intensive phase)
The primary analysis used a subset of records from 3750 patients from 42 observational studies; of these patients, 2720 were treated with an individualized MDR-TB regimen and 1030 were treated with standardized MDR-TB regimens. Of the 13 104 records in the main IPD, 9354 records were excluded for the following reasons: lost to follow-up (n=2261), died (n=2043), did not receive an injectable (n=1094), no information on duration of injectable (n=2341), number of medicines likely to be effective less than five or less than four plus pyrazinamide (n=1450) and duration of injectable longer than 20 months (n=165).
PICO question 6–2018 (MDR/RR-TB, 2018) (on regimen duration)
The evidence to inform PICO question 6–2018 (MDR/RR-TB, 2018) was derived from a subset of 6356 patients from 51 observational studies for the primary analysis. Of the 6356 patients, 5352 were treated with an individualized MDR-TB regimen and 1004 were treated with a standardized MDR-TB regimen. Of the 13 104 records in the main IPD, 6748 records were excluded for the following reasons: lost to follow-up (n=2261), died (n=2043), treatment duration not available (n=230), number of effective drugs less than five or less than four plus pyrazinamide (n=2072), treatment duration less than 6 months (n=52) and treatment duration more than or equal to 36 months (n=90).
PICO question 7–2018 (MDR/RR-TB, 2018) (on treatment duration after culture conversion)
The analysis to address PICO question 7–2018 (MDR/RR-TB, 2018) was derived from a subset of 4175 patients from 39 observational studies. All but three of the 4175 patients were on individualized regimens. The reasons for exclusion of 8929 records from the main dataset were as follows: lost to follow-up (n=2261), died (n=2043), treatment duration not reported (n=230), culture information not reported (n=1945), baseline culture negative (n=754), patient never culture converted (n=426), number of effective drugs less than five or less than four plus pyrazinamide (n=1215), treatment duration less than 6 months (n=4), treatment duration more than or equal to 36 months (n=49) and culture converted post-treatment (n=2).
PICO question 1–2019 (MDR/RR-TB, 2019) (use of bedaquiline longer than 6 months)
To analyse treatment success, failure, relapse and death for the use of bedaquiline longer than 6 months, the data were derived from the endTB observational study, with the overall dataset comprising a total of 1094 patients from 13 countries (77). ³⁹ The data analysed to answer this question were patients from the endTB observational study cohort who received bedaquiline for at least 6 months, had started bedaquiline within the first month of the treatment episode and did not receive delamanid concomitantly with bedaquiline during treatment; among patients with treatment success, data were from those who received at least 17.5 months of treatment overall. A total of 515 patients met these criteria. The intervention group comprised 242 patients who received bedaquiline for more than 203 days⁴⁰ overall, and they were compared to 273 patients who received bedaquiline for a total of 168–203 days. Additional data sources considered by the GDG 2019 included a cohort of 112 patients from Belarus treated with bedaquiline (of whom two had inadequate treatment information and were excluded), and a cohort of 123 patients from an MSF-managed clinic in Uzbekistan treated with bedaquiline (with one patient excluded due to inadequate treatment information). Of these 232 eligible patients, 65 received bedaquiline for more than 203 days and 72 received bedaquiline for 168–203 days. The primary analyses featured the endTB observational study data only.
PICO question 2–2019 (MDR/RR-TB, 2019) (use of bedaquiline and delamanid together)
To analyse treatment success, failure, relapse and death for the concurrent use of bedaquiline and delamanid, the data were derived from the same cohort of patients from the endTB observational study that informed PICO question 1–2019. However, in this dataset, only 92 patients received both medicines together for any period of time, and even fewer started bedaquiline and delamanid at the same time and within the first month of treatment (n=35). Another three patients were receiving concomitant bedaquiline and delamanid by the end of the first month of treatment, bringing the total number to 38. The remaining 57 patients started the two medicines more than 30 days apart and were therefore not included. Additional data sources comprised a cohort of 100 patients treated with bedaquiline in Mumbai, India (from an MSF-supported project), of whom 86 received some form of concomitant treatment with bedaquiline and delamanid during therapy; 62 of these 86 initiated the two medicines within 30 days of each other, and 46 of these 62 began both medicines during the first month of their treatment episode. The total intervention population therefore comprised 84 patients: 38 from the endTB observational study cohort and 46 from the Mumbai dataset. Because the data available were limited, the sources of data for the comparator populations were derived from the endTB observational study, and the datasets from Belarus, Mumbai and Uzbekistan. There were inadequate numbers of patients available in the IPD for any meaningful analyses (n=4 patients who received bedaquiline and delamanid together). The primary comparison group included 401 patients (n=302 from the endTB observational study, n=82 from the Belarus dataset, n=17 from the Uzbekistan dataset and n=0 from the Mumbai dataset). These patients initiated bedaquiline within the first month of treatment and did not receive bedaquiline beyond 6 months duration. The secondary comparison group was derived from the endTB observational study and comprised 102 patients who received delamanid within the first month of treatment and who did not receive an extended duration of delamanid. No patients in the datasets from Belarus, Mumbai or Uzbekistan received delamanid for this duration. The median duration of concurrent use of bedaquiline and delamanid among the 84 patients in the intervention group was 18.5 months (IQR: 9 months, 21 months).
Additional data presented included safety data from the DELamanId Bedaquiline for ResistAnt TubErculosis (DELIBERATE) trial (AIDS Clinical Trials Group A5343). The DELIBERATE trial is a randomized, open-label, three-arm pharmacokinetic and safety trial conducted at study sites in Peru and South Africa. Eligible patients were aged 18 years and older, with pulmonary MDR-TB (or rifampicin monoresistance) receiving treatment for MDR-TB, but without clofazimine, and with moxifloxacin replaced by levofloxacin and a baseline QTcF of less than 450 ms. In addition to the MDR-TB treatment regimen with the conditions described above, the regimens used in the three study arms comprised the addition of bedaquiline 400 mg once daily for 2 weeks, then 200 mg thrice weekly for 22 weeks; the addition of delamanid 100 mg twice daily for 24 weeks; and the addition of both bedaquiline and delamanid. The primary objective of the trial was to compare the mean change from baseline in QTcF (averaged over weeks 8–24) when bedaquiline and delamanid were co-administered with the mean change observed when each drug was administered alone.
In addition to the data reviewed for PICO questions 1–2019 and 2–2019, the GDG 2019 was provided with and reviewed data from a study in South Africa on the use of bedaquiline during pregnancy. This observational cohort study included information from 108 pregnant women with RR-TB who were recruited from one MDR/RR-TB referral hospital in South Africa between January 2013 and December 2017. As part of their MDR/RR-TB regimen, 58 women received bedaquiline; they were compared with 50 women who had no bedaquiline in their regimen. The women in this study gave birth to 109 live infants, of whom 49 had bedaquiline exposure in utero and 60 had no bedaquiline exposure in utero. Clinical assessments were carried out at 2, 6 and 12 months after birth to document infant outcomes. The main objective of the study was to document treatment, pregnancy and infant outcomes among women treated for RR-TB with second-line TB drugs during pregnancy.
When reviewing evidence and formulating the recommendations, the GDG 2019 considered the need for the guidelines to also cater to key subgroups that were not well represented in the 2018 IPD meta-analysis – notably, children. Where data on children were unavailable, evidence from adults was extrapolated to children. The best available evidence was used to construct recommendations for a regimen that has high relapse-free cure rates, and that reduces the likelihood of death and the emergence of additional resistance while minimizing harms. The GDG 2019 was aware of the paediatric MDR-TB IPD meta-analysis on 975 clinically diagnosed or bacteriologically confirmed pulmonary or extrapulmonary TB cases that was used for the 2016 treatment recommendations (71). Children with XDR-TB (pre-2021 definition) were excluded from that analysis (n=36) because their treatment regimens were not considered to be comparable with those of other MDR-TB patients, and their numbers were too low to be analysed independently. No RCTs were included (or known to exist) at the time this dataset was compiled, and the overall certainty in the estimates of effect based on this evidence was judged to be very low. However, in July 2019, preliminary data from the DELIBERATE trial were made available to the GDG 2019 to partly address PICO question 9; the overall certainty in the estimates of effect for this study was judged to be low.
PICO question 1–2021 (Childhood TB, 2021) (use of bedaquiline in MDR/RR-TB patients aged below 6 years)
To answer the PICO question on the use of bedaquiline in children aged below 6 years, data from two Phase 2 trials (TMC207-C211 and IMPAACT P1108) were reviewed by the GDG 2021. TMC207-C211 is a Phase 2, open-label, single-arm study to evaluate the pharmacokinetics, safety, tolerability and anti-mycobacterial activity of bedaquiline in combination with a background regimen of MDR-TB medications for the treatment of children and adolescents aged 0–17 years who have bacteriologically confirmed or clinically diagnosed pulmonary and selected forms of extrapulmonary MDR-TB.⁴¹ IMPAACT P1108 is a Phase 1–2 dose finding modified age de-escalation study to evaluate the pharmacokinetics, safety and tolerability of bedaquiline in combination with optimized individualized MDR-TB regimens in children living with HIV and HIV-uninfected children with clinically diagnosed or confirmed pulmonary (intrathoracic) and selected forms of extrapulmonary MDR-TB.⁴²
Data reviewed from TMC207-C211 corresponded to children aged 5–18 years and data from IMPAACT P1108 included children aged 0–6 years; therefore, the review of pharmacokinetics and safety data focused mainly on data from IMPAACT P1108. Although the sample size of the available interim data for review was small (n=12), the GDG 2021 concluded that in children aged 0–6 years, cardiac safety signals were not distinct from those reported in adults. Population pharmacokinetic models from both studies suggest that drug exposures observed in adults can be reached in most children receiving bedaquiline, although some dose modification may be necessary depending on the age and weight of the child.
In addition, data from a paediatric MDR/RR-TB IPD were analysed descriptively (24 231 records from all six WHO regions, the majority from India and South Africa). The search was conducted in April 2020. Just under 20 000 of these records were used for a matched analysis of treatment outcomes in children being treated for DR-TB. The analysis included 40 children aged below 6 years and 68 children aged 6–12 years who received bedaquiline. In the matched analysis, bedaquiline was significantly associated with shorter treatment duration and a lower aOR of injectable TB drug use. There was no statistically significant difference in successful treatment outcomes between children aged below 6 years receiving an all-oral bedaquiline-based regimen and those not receiving bedaquiline (89% versus 97%, P=0.9). Residual confounding (including confounding by indication) was thought to be likely.
A child-friendly formulation of bedaquiline (20 mg scored uncoated tablet) is being used in the Janssen C211 study to dose children aged below 5 years and will also soon be used in an updated protocol of the IMPAACT study P1108 (to date, this study has used the 100 mg formulation in all age groups). No head-to-head studies were conducted to examine the bioequivalence of the 20 mg and the 100 mg formulation of bedaquiline. Indirect bioequivalence testing showed that both tablets have the same bioavailability and can be used interchangeably at the same total dose. Findings from the bedaquiline crush study (78) also showed that the bioavailability of bedaquiline tablets suspended in water was the same as for tablets swallowed whole.
PICO question 2–2021 (Childhood TB, 2021) (use of delamanid in MDR/RR-TB patients aged below 3 years)
To answer the PICO question on the use of delamanid in children aged below 3 years, data were reviewed by the GDG 2021 from a Phase 1, open-label, age de-escalation trial designed to assess the pharmacokinetics, safety and tolerability of delamanid administered twice daily for 10 days in children with MDR/RR-TB on treatment with an optimized background regimen (protocol 242–12–232)⁴³ and from the corresponding open-label extension study (protocol 242–12–233).⁴⁴ Data from cohorts 1 (age 12–17 years), 2 (age 6–11 years), 3 (age 3–5 years) and 4 (age 0–2 years) for both protocols were reviewed. Exposures in the 0–2-year age group were lower than those of children aged 3 years and older, necessitating a modelling or simulation approach to dosing. No cardiac safety signals distinct from those reported in adults were observed in children aged 0–2 years. However, consideration of these findings should take into account that children had lower drug exposures than adults. Pharmacodynamic simulations suggested that clinically meaningful changes in QT (i.e. prolongation) would be unlikely in children aged below 3 years, even if higher doses were used to reach drug exposures comparable to those achieved in adults.
CNS effects (paraesthesia, tremors, anxiety, depression and insomnia) were included in the delamanid label for both adults and children as important potential safety concerns for the drug. In March 2021, the study sponsor released a statement of intent to modify the labelling to include hallucinations as an adverse reaction. This new safety signal has been more prevalent among children than adults, with 15 reports in 14 children aged 2–16 years in India, the Philippines, South Africa, Tajikistan and Ukraine.
Children experiencing this safety signal included some with extensively resistant forms of TB (MDR/XDR-TB) treated with delamanid under programmatic conditions (12 reports) and children enrolled in a clinical trial studying delamanid for TB prevention (3 reports). Seven of the 15 reports were for children also receiving cycloserine (under programmatic conditions). The GDG noted the importance of side-effects involving the CNS in young children, considering their dynamic brain development.
In addition to data from the trials, data from a paediatric DR-TB IPD were analysed descriptively (24 231 records from all six WHO regions, the majority from India and South Africa). The search was conducted in April 2020. Just under 20 000 of these records were used for a matched analysis of treatment outcomes in children being treated for DR-TB. The paediatric DR-TB IPD included only seven children aged below 3 years treated with delamanid, 14 children aged 3–6 years and 69 children aged 6–12 years. All 21 children aged below 6 years were successfully treated. The number of children was insufficient for a matched analysis.
Remarks
The GDG 2018 assessed the individual contribution to patient outcomes of medicines used in longer MDR-TB regimens, using primarily the estimates of effect from the 2018 IPD meta-analysis and Trial 213 (delamanid) for PICO question 3–2018 (MDR/RR-TB, 2018) (see Annex 5 for the respective GRADE summaries of evidence for each medicine, and the evidence-to-decision framework). Following a thorough assessment of the relative benefits and harms, recommendations were made for each medicine and they were classified into three groups (see Table 3.1, Table 3.2 and Table 3.3).
- Group A: fluoroquinolones (levofloxacin and moxifloxacin), bedaquiline and linezolid were considered highly effective and strongly recommended for inclusion in all regimens unless contraindicated.
- Group B: clofazimine and cycloserine or terizidone were conditionally recommended as agents of second choice.
- Group C: included all other medicines that can be used when a regimen cannot be composed with Group A or Group B agents. The medicines in Group C are ranked by the relative balance of benefit to harm usually expected of each.
Other medicines that are not included in Groups A–C are as follows:
- Kanamycin and capreomycin – these medicines were associated with poorer outcomes when used; therefore, they are no longer recommended for use in MDR-TB regimens.
- Gatifloxacin and high-dose isoniazid, and thioacetazone – gatifloxacin and high-dose isoniazid were used in only a few patients, and thioacetazone was not used at all. Currently, quality-assured preparations of gatifloxacin are not available, following its withdrawal from the market due to concerns about dysglycaemias. Thioacetazone is unlikely to have a role in contemporary longer regimens and is not currently available in a quality-assured formulation. High-dose isoniazid may have a role in patients with confirmed susceptibility to isoniazid (see Subgroup considerations).
- Clavulanic acid – this medicine should be included in MDR/RR-TB regimens only as a companion agent to the carbapenems (imipenem–cilastatin and meropenem). When used in this way, it should be given with every dose of carbapenem, and should not be counted as an additional effective TB agent.
No recommendation on perchlozone, interferon gamma or sutezolid was possible owing to the absence of final patient treatment outcome data from appropriate patient studies.
Regarding the use of bedaquiline in patients aged below 18 years, and considering that exposure– response (efficacy) profiles can be extrapolated from adults to children, the GDG concluded that the doses evaluated in children and adolescents in two trials (Phase 2 trial TMC207-C211 and Phase 1–2 IMPAACT P1108; see Annex 6) do not appear to result in exposures that would put patients aged 6–17 years at increased risk for treatment failure. The safety risk in children aged 6 years and older enrolled in the trials – all of whom were HIV-negative and had limited exposure to other QT intervalprolonging medications – did not appear to exceed that of adults. The variability present in the limited sample size precluded a comment on exposure–response (safety). The GDG 2018 also concluded that the risk–benefit considerations for the use of bedaquiline in patients aged 6–17 years are similar to those considered for adults; however, the GDG stressed the need for more data before considering upgrading this recommendation to “strong”.
The GDG review in 2021 determined that the balance between desirable and undesirable effects probably favours the use of bedaquiline in children aged below 6 years. The GDG 2021 highlighted that the benefits may vary depending on specific contexts and population characteristics, such as by nutritional status. The GDG also noted that the potential higher cost of bedaquiline in an MDR/RR-TB treatment regimen should be considered in the context of the benefits of shorter injectablefree regimens (i.e. less travel, reduced time spent in clinics and fewer AEs). In addition, they judged that equity might increase when bedaquiline becomes available to younger children, because its use would be acceptable to most stakeholders, and that one of the main feasibility aspects would be related to the need for safety monitoring (i.e. access to ECG monitoring, as well as staff capacity for monitoring). However, the panel judged that implementing the use of bedaquiline in young children was probably feasible.
With respect to the use of delamanid in children aged below 6 years, the GDG review in 2018 decided that – based on findings in adults, and on the pharmacological and safety data reviewed – extrapolations on efficacy and safety should be restricted to children aged 3–5 years, but not to children aged below 3 years (see Annex 5). Exposure profiles in children aged 3–5 years were comparable to adults, and were no higher than in children aged 6 years and older, for whom past GDGs convened by WHO had already recommended the use of delamanid (15, 26). Based on the laboratory and cardiac data provided, no safety signals distinct from those reported in adults were observed in children aged 3–5 years. The GDG nonetheless had concerns about the feasibility of administering the correct dose to children aged 3–5 years, given that the special formulation used in the trial (25 mg) would not be available in the foreseeable future, and that only the adult tablet (50 mg) is available, which is not bioequivalent and presents challenges to manipulating its contents without compromising its effectiveness.
The GDG review in 2021 concluded that the balance between desirable and undesirable effects probably favours the use of delamanid in children aged below 3 years. The GDG 2021 further stated that when the 25 mg dispersible tablet became available in the future, the resource implications could vary. It was thought that delamanid containing longer treatment regimens could potentially increase equity and be acceptable to stakeholders. In addition, the GDG 2021 judged that it would probably be feasible to use delamanid in children of all ages, especially as the child-friendly formulation of delamanid was expected to become available later in 2021 (this formulation is now available). This judgement also considered that adult tablets cannot be split, crushed or dissolved to ease administration in children without potentially altering bioavailability.
As a result of these multiple reviews as new data have gradually become available, the use of bedaquiline and delamanid are no longer restricted by the age of the patient.
Table 3.1. Grouping of medicines recommended for use in longer MDR-TB regimensᵃ
DST: drug susceptibility testing; ECG: electrocardiogram; GDG: Guideline Development Group; IPD: individual patient data; LPA: line probe assay; MDR-TB: multidrug-resistant TB; TB: tuberculosis.
a This table is intended to guide the design of individualized, longer MDR-TB regimens (the composition of the recommended shorter MDR-TB regimen is largely standardized; see Treatment of drug-resistant TB using 9-month regimens). Medicines in Group C are ranked by decreasing order of usual preference for use, subject to other considerations. The 2018 IPD meta-analysis for longer regimens included no patients on thioacetazone and too few patients on gatifloxacin and high-dose isoniazid for a meaningful analysis. No recommendation on perchlozone, interferon gamma or sutezolid was possible owing to the absence of final patient treatment outcome data from appropriate studies (see Annex 6).
b Bedaquiline is usually administered at 400 mg orally once daily for the first 2 weeks, followed by 200 mg orally three times per week for 22 weeks (total duration of 24 weeks). As a result of multiple reviews as new data have gradually become available, the use of bedaquiline is no longer restricted by the age of the patient. Evidence on the safety and effectiveness of bedaquiline use beyond 6 months was insufficient for review in 2018. Therefore, the use of bedaquiline beyond 6 months was implemented following best practices in “off-label” use (79). New evidence on the safety profile of bedaquiline use beyond 6 months was available to the GDG 2019, but the GDG was not able to assess the impact of prolonged bedaquiline use on efficacy, owing to the limited evidence and potential residual confounding in the data. However, the evidence supports the safe use of bedaquiline beyond 6 months in patients who receive appropriate schedules of baseline and follow-up monitoring. The use of bedaquiline beyond 6 months remains as off-label use and, in this regard, best practices in off-label use still apply.
c Evidence on the concurrent use of bedaquiline and delamanid was insufficient for review in 2018. In 2019, new evidence on the concurrent use of bedaquiline and delamanid was made available to the GDG. Regarding safety, the GDG concluded that the data suggest no additional safety concerns regarding concurrent use of bedaquiline and delamanid. Both medicines may be used concurrently in patients who have limited other treatment options available to them, provided that sufficient monitoring (including baseline and follow-up ECG and electrolyte monitoring) is in place. The data on the effectiveness of concurrent use of bedaquiline and delamanid were reviewed by the GDG; however, owing to the limited evidence and potential residual confounding in the data, the GDG was unable to proceed with a recommendation on effectiveness.
d Use of linezolid for at least 6 months was shown to increase effectiveness, although toxicity may limit use. The analysis suggested that using linezolid for the entire duration of treatment would optimize its effect (about 70% of patients on linezolid with data received it for >6 months and 30% for 18 months or the entire duration). No patient predictors for early cessation of linezolid could be inferred from the IPD subanalysis.
e Evidence on the safety and effectiveness of delamanid beyond 6 months was insufficient for review. The use of delamanid beyond these limits should follow best practices in “off-label” use (79). As a result of multiple reviews as new data have gradually become available, the use of delamanid is no longer restricted by the age of the patient.
f Pyrazinamide is counted as an effective agent only when DST results confirm susceptibility.
g Every dose of imipenem–cilastatin and meropenem is administered with clavulanic acid, which is available only in formulations combined with amoxicillin. Amoxicillin–clavulanic acid is not counted as an additional effective TB agent and should not be used without imipenem–cilastatin or meropenem.
h Amikacin and streptomycin are to be considered only if DST results confirm susceptibility, and if high-quality audiometry monitoring for hearing loss can be ensured. Streptomycin is to be considered only if amikacin cannot be used (i.e. if it is unavailable or there is documented resistance) and if DST results confirm susceptibility (i.e. resistance to streptomycin is not detectable with second-line molecular LPAs and phenotypic DST is required). Kanamycin and capreomycin are no longer recommended for use in MDR-TB regimens.
i These agents showed effectiveness only in regimens without bedaquiline, linezolid, clofazimine or delamanid, and are thus proposed only when other options to compose a regimen are not possible.
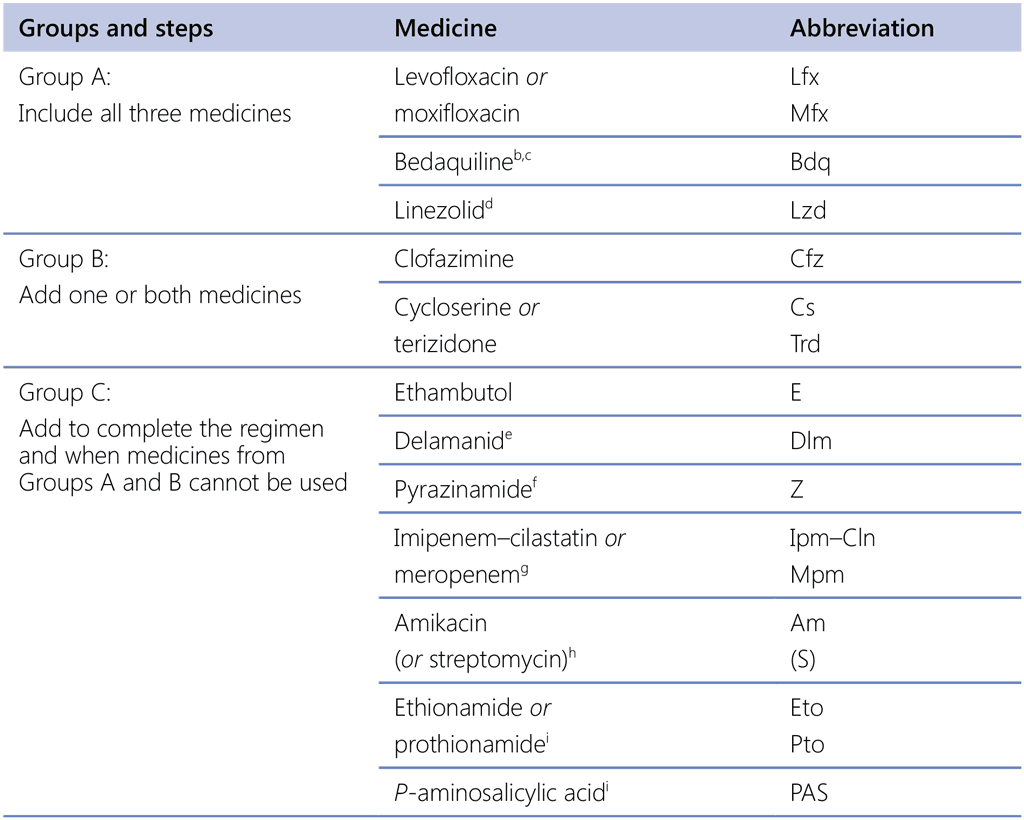
Table 3.2. Relative risk for treatment failure or relapse, and death (versus treatment success), 2018 IPD meta-analysis for longer MDR-TB regimens and delamanid Trial 213 (intent-to-treat population)ᵃ
CL: confidence limits; GDG: Guideline Development Group; IPD: individual patient data; MDR-TB: multidrug-resistant tuberculosis.
a See also text, Table 3.3 and Annex 5 and Annex 6 for more detail on how the estimates were derived and the additional factors considered by the GDG when reclassifying medicines for use in longer MDR-TB regimens, as shown in Table 3.1.
b The values are the unadjusted risk ratios, as defined by the study investigators of Trial 213 by month 24.
PICO question 4–2018 (MDR/RR-TB, 2018) (number of agents likely to be effective)
Regarding PICO question 4–2018 (MDR/RR-TB, 2018), the analysis showed that in longer MDR-TB treatment regimens, the risk of treatment failure, relapse and death was comparable when the treatment started with four, five or six medicines that were likely to be effective. It also showed that patients who took three agents in the continuation phase – the situation expected when starting with four agents and stopping the injectable agent at the end of the intensive phase – fared no worse than those who took four agents in the continuation phase.
Given that drug–drug interactions, pill burden and likelihood of AEs all increase with the number of agents in a regimen, it would be desirable to give patients the minimum number of medicines necessary to obtain comparable levels of relapse-free cure. When deciding on the minimum number of agents to recommend, the GDG 2018 considered analyses that included injectable agents in the regimens, while fully cognizant that future longer regimens are expected to be increasingly injectable free. Moreover, it was important to provide for situations in which more than one medicine is stopped at some point during treatment, either because of its indication for use – bedaquiline and delamanid on-label use is 6 months – or because of tolerability (particularly linezolid; Table 3.3) (80); hence, for most of its duration, the regimen would contain two key agents fewer than at the start. Although bedaquiline use beyond 6 months is referred to as off-label use, new evidence on the safety profile of bedaquiline use beyond 6 months was available to the GDG 2019. This evidence supports the safe use of bedaquiline beyond 6 months in patients who receive appropriate schedules of baseline and follow-up monitoring. The use of bedaquiline beyond 6 months continues to be off-label use; thus, best practices in off-label use still apply.
The 2018 IPD included experience from more than 300 patients who were treated with linezolid for at least 1 month, mostly at a dose of 600 mg/day, with information on duration of use. About 30% only received linezolid for 1–6 months, but more than 30% received it for more than 18 months, and these patients had the lowest frequency of treatment failure, LTFU and death. A plot of linezolid duration and treatment failure suggests that the optimal duration of use would be about 20 months, corresponding to the usual total duration of a longer MDR-TB regimen. However, such an analysis does not account for survivorship bias, meaning that those who complete the full length of treatment are more likely to have a successful outcome, given that deaths and losses to follow-up occur earlier. No clear pattern could be discerned for type of AE and duration of use, although a few cases were reported with optic neuropathy, known to be associated with long-term use of linezolid (81), whereas haematological toxicity was reported regardless of duration of use.
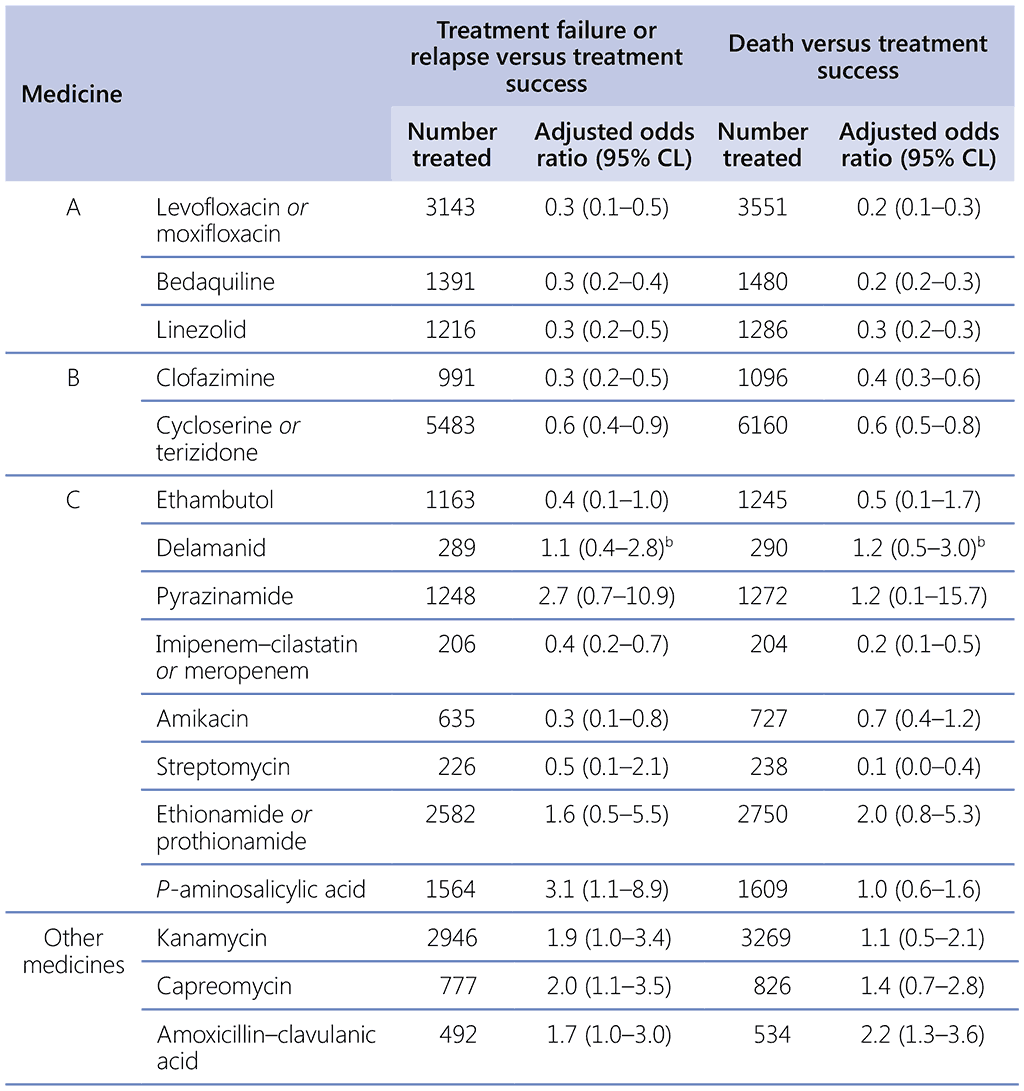
Table 3.3. Serious AEs in patients on longer MDR-TB regimensᵃ
GDG: Guideline Development Group; IPD: individual patient data; MDR-TB: multidrug-resistant TB; TB: tuberculosis.
a From an “arm-based network” meta-analysis of a patient subset from the 2016 IPD for which AEs resulting in permanent discontinuation of a TB medicine (27 studies) or classified as Grade 3–5 (three studies) were reported. There are slight differences between the final estimates cited in the resultant publication (80) and the values derived at the time of the GDG and shown in this table, because an expanded dataset was used in the publication; however, the slight differences have no impact on the conclusions drawn on the use of these medicines. There were insufficient records on delamanid, imipenem–cilastatin and meropenem to estimate risks. Agents that are not in Groups A, B or C are italicized.
In 2018, the GDG recommended that, where possible, regimens be composed of all three Group A agents and at least one Group B agent, so that treatment starts with at least four medicines likely to be effective, and that at least three agents are continued for the remaining duration of treatment if bedaquiline is stopped after 6 months. New evidence on the safety profile of bedaquiline use beyond 6 months was available to the GDG 2019. This evidence supports the safety of using bedaquiline beyond 6 months in patients who receive appropriate schedules of baseline and follow-up monitoring. If only one or two Group A agents can be used, both Group B agents are included. If the regimen cannot be composed with agents from Groups A and B alone, Group C agents are added to complete it. For patients in whom two agents from Group A are more likely to be stopped before the end of treatment (e.g. pre-existing comorbidities require that both bedaquiline and linezolid be stopped early because of health risks), then starting with five effective agents rather than four may be advisable. These provisions are expected to apply to most MDR/RR-TB patients, including those with additional resistance to fluoroquinolones or other medicines.
PICO question 8–2019 (MDR/RR-TB, 2019) (use of bedaquiline longer than 6 months)
Regarding PICO question 8–2019 (MDR/RR-TB, 2019), the analysis yielded aORs of 1.5 (95% CI: 0.7–2.7) for treatment success versus failure, 0.8 (95% CI: 0.2–0.4) for treatment success versus death, 1.0 (95% CI: 0.5–1.7) for treatment success versus failure or death, and 0.8 (95% CI: 0.5–1.2) for treatment success versus all unfavourable outcomes. The evidence reviewers had planned to use two analytical approaches designed to minimize bias; that is, marginal structural models to account for time-varying confounders, and for exact and propensity score matching of patient characteristics. However, sample size meant that there were limitations in how the first approach could be applied; also, owing to limitations with the dataset, biostatisticians advised that it was not possible to adjust for confounders according to the original data analysis plan. The GDG 2019 noted that the population included in the studies that were assessed was highly selected, with the potential for confounding by indication (i.e. the people who received bedaquiline for >6 months were likely to have done so because of clinical factors that indicated prolonged treatment with bedaquiline). The GDG concluded that there was a high likelihood of residual confounding in the data, and that the patient population addressed in the study did not permit extrapolation to routine use in all MDR/RR-TB patients. This precluded a formal recommendation on the efficacy or effectiveness of bedaquiline use beyond 6 months duration; however, the GDG 2019 concluded that a statement on safety could be made. This information is included in Implementation considerations and in a table note for Table 3.1.
Regarding AEs, among the 750 patients receiving bedaquiline without concomitant delamanid in the endTB observational study (total exposure of 6316 person-months), 26 patients experienced a drug-related AE (rate: 0.44 per 100 person-months of exposure), with 16 patients having this event classified as a serious AE (rate: 0.25 per 100 person-months of exposure). In the first 203 days of exposure to bedaquiline (total exposure of 4304 person-months), 20 of the 26 drug-related AEs and 15 of the 16 serious AEs occurred; the remaining six of the 26 drug-related AEs and one of the 16 serious AEs occurred subsequently. All patients who received bedaquiline for more than 203 days did not experience a drug-related AE (of any grade) in the first 203 days of treatment. Also, rates of treatment drug-related AEs appeared to be lower after the first 203 days – at 0.51 in the first 203 days versus 0.30 in the subsequent days per 100 person-months. Similarly, rates of drug-related serious AEs appeared to be lower after the first 203 days – at 0.35 in the first 203 days versus 0.05 in the subsequent days per 100 person-months.
QTcF values among people receiving bedaquiline increased by an average of 22 ms (from 397 ms to 419 ms) from those taken before or at the time of first receipt of bedaquiline to the end of the first month. In subsequent months of exposure, the mean QTcF values were all lower than at the end of the first month (range: 404–419 ms). Increases in QTcF of more than 60 ms from baseline occurred in about 12% of patients. QTcF prolongation of more than 500 ms was rare, occurring in 0.4–1.5% of patients during each of the first 9 months, but not thereafter. The greatest number of occurrences of QTcF of more than 500 ms happened among people receiving bedaquiline and clofazimine; however, this was also the most common combination of medicines received.
Drug-related cardiac AEs occurred in 22 people; of these, 15 were among people receiving bedaquiline with clofazimine, but no moxifloxacin or delamanid (rate: 0.3 per 100 person-months), five were among people receiving bedaquiline with clofazimine and moxifloxacin, but no delamanid (rate: 0.3 per 100 person-months), and two were among people receiving bedaquiline and delamanid, regardless of clofazimine and moxifloxacin use (rate: 0.2 per 100 person-months). No events occurred among people receiving bedaquiline without clofazimine, moxifloxacin and delamanid.
Regarding bedaquiline exposure during pregnancy, the findings of the cohort study demonstrated no statistically significant differences in birth or pregnancy outcomes when comparing infants who had intrauterine bedaquiline exposure with those who did not have this exposure (P=0.741 for birth outcomes and P=0.312 for pregnancy outcomes) (61). There were 45 live births (92% of total) in the bedaquiline exposed group compared with 54 live births (90% of total) in the unexposed group. In addition, there were four fetal and neonatal deaths in the infants exposed to bedaquiline (8% of the total bedaquiline exposed group, with three stillbirths and one termination of pregnancy) and six fetal and neonatal deaths in the bedaquiline unexposed group (10% of the total unexposed group, comprising three stillbirths and three miscarriages) (61). The results of the study also demonstrated that treatment outcomes were favourable for pregnant women exposed to bedaquiline compared with those not exposed (71% vs 62%, respectively, P=0.349) (61). Pregnancy outcomes included live births and unfavourable pregnancy outcomes (fetal and neonatal deaths, preterm births <37 weeks and low birth weight <2500 g); infant outcomes included weight gain and developmental milestones and the diagnosis of TB (61). Of all pregnancy and infant outcomes assessed, only low birth weight was associated with bedaquiline exposure in utero (45% vs 26%, P=0.034). The average weight in bedaquiline exposed infants was 2690 g versus 2900 g in infants not exposed to bedaquiline. However, it was not possible to conclusively ascribe this effect to bedaquiline, and more investigation is needed to explore this relationship (61). There were no significant differences in infant growth after birth: in a subanalysis of 86 babies followed up prospectively – 41 exposed to bedaquiline in utero and 45 not exposed – 88% of babies exposed to bedaquiline in utero had normal weight gain at 1 year of age versus 82% of babies not exposed (P=0.914) (61).
PICO question 9–2019 (MDR/RR-TB, 2019) (use of bedaquiline and delamanid together)
Regarding PICO question 9 (MDR/RR-TB, 2019), the analyses yielded aORs of 1.6 (95% CI: 0.5–5.4) for treatment success versus treatment failure, 0.8 (95% CI: 0.3–2.1) for treatment success versus death, 1.2 (95% CI: 0.6–2.5) for treatment success versus failure or death, and 0.6 (95% CI: 0.3–1.1) for treatment success versus all unfavourable outcomes. Regarding AEs, among the 92 patients receiving bedaquiline with concomitant delamanid during treatment in the endTB observational study (total exposure of 1095 person-months), two bedaquiline-related AEs and delamanid-related AEs occurred (combined rate: 0.46 per 100 person-months of exposure). This rate was comparable to the rates among people receiving bedaquiline alone (0.41 per 100 person-months of exposure) and delamanid alone (0.68 per 100 person-months of exposure). Two drug-related serious AEs occurred among the 92 patients receiving concomitant bedaquiline and delamanid, one attributed to each drug (combined rate: 0.09 per 100 person-months of exposure). The rate of these events was lower than the rates of drug-related serious AEs among patients receiving either of these drugs alone (bedaquiline, 0.28; delamanid, 0.39). No fatal drug-related events occurred among patients receiving bedaquiline and delamanid concurrently.
QTcF values among people receiving bedaquiline and delamanid increased by an average of 15 ms (from 398 ms to 413 ms) from those taken before or at the time of first receipt of concurrent bedaquiline and delamanid use, to the end of the first month. In subsequent months of exposure, the mean QTcF values were similar to those at the end of the first month (range: 404–420 ms). QTcF prolongation of more than 500 ms was rare, occurring in only one patient in month 7 of concomitant exposure. Drug-related cardiac AEs were infrequent, occurring in only two of 92 people exposed to concomitant bedaquiline and delamanid (rate: 0.2 per 100 person-months). Only one drug-related cardiac serious AE occurred (rate: 0.1 per 100 person-months). No fatal drug-related cardiac events occurred among the 92 people exposed to bedaquiline and delamanid concurrently.
In the endTB observational study overall (n=1094), there were two fatal drug-related cardiac events (sudden deaths attributable to QT prolongation), and one other patient experienced a cardiac arrhythmia. The two deaths occurred among patients receiving bedaquiline, clofazimine, capreomycin and p-aminosalicylic acid (but not moxifloxacin or delamanid); in both patients, hypokalaemia was present. These patients were not included in the analysis related to this PICO question because they did not meet the criteria for inclusion according to the predefined statistical analysis plan. However, recognizing that these estimates of serious AEs were absolute and not relative, the panel felt that this additional evidence was important for close monitoring when the final data of the endTB observational study become available.
The GDG agreed that there was insufficient evidence to assess the efficacy or effectiveness of the concomitant use of bedaquiline and delamanid, given that there were only 84 patients in the intervention group and the data did not lend themselves to a meaningful analysis for the secondary comparator (extended use of delamanid alone) because the populations were too different to allow for the matching that is usually carried out. This precluded a formal recommendation on the efficacy or effectiveness of the concomitant use of bedaquiline and delamanid; however, the GDG concluded that a statement on safety could be made. This information is included in Implementation considerations and in a table note for Table 3.1.
Additional data presented from the DELIBERATE trial highlighted that – among the patients randomized to bedaquiline (n=28), delamanid (n=27) or both medicines (n=27) – the on-treatment change in QTcF from baseline was 11.9 ms, 8.6 ms and 20.7 ms, respectively.⁴⁵ Of the 27 patients who received both medicines, 10 (37.0%) experienced a Grade 1⁴⁶ QT prolongation AE, and two (7.4%) experienced a Grade 2 QT AE. In the bedaquiline arm, 32.0% and 3.6% of patients experienced Grade 1 and 2 QT AEs; in the delamanid arm, these figures were 41.0% for a Grade 1 QT adverse event and 7.4% for a Grade 2 QT adverse event. No patients experienced Grade 3 or 4 QT adverse events. The study investigators concluded that the QTcF prolongation effects of concurrent delamanid and bedaquiline use were not greater than their additive effects. The GDG noted that the QT adverse events in the DELIBERATE trial were surrogate markers of sudden cardiac death. They also noted that levofloxacin was the fluoroquinolone of choice in regimens given to patients in the DELIBERATE trial and that serum potassium was closely monitored.
PICO question 1–2021 (Childhood TB 2021) (use of bedaquiline in MDR/RR-TB patients aged below 6 years) and PICO question 2–2021 (Childhood TB 2021) (use of delamanid in MDR/RR-TB patients aged below 3 years)
Regarding PICO question 1–2021 (Childhood TB 2021) and PICO question 2–2021 (Childhood TB 2021), the details of the evidence review and GDG deliberations can be found in Module 5. Management of tuberculosis in children and adolescents.
Subgroup considerations
MDR/RR-TB alone or with additional resistance
A longer regimen is used where a shorter regimen cannot be used; it is more likely to be effective if its composition is guided by reliable information on drug susceptibility. The design of longer regimens for MDR/RR-TB patients with additional resistance follows a similar logic to that used for other MDR/RR-TB patients. All MDR/RR-TB patients should be tested for resistance to fluoroquinolones as a minimum before starting MDR-TB treatment. If the use of amikacin is being considered in the regimen, then rapid testing for second-line injectable agents should be performed. Other tests that may help to inform regimen choice and composition are those for resistance to agents such as bedaquiline, delamanid, linezolid and pyrazinamide, and for mutation patterns commonly associated with resistance to isoniazid and ethionamide or prothionamide. In many settings, DST for other medicines commonly used for MDR-TB treatment is not usually reliable enough to guide regimen composition. Because of this, other elements may be necessary to determine the likelihood of effectiveness (see Implementation considerations). NTPs should possess or rapidly build the capacity to undertake DST, and all efforts should be made to ensure access to approved rapid molecular tests. Until the capacity for second-line DST – including for bedaquiline, linezolid and clofazimine – becomes available, treatment decisions may need to rely on the likelihood of resistance to medicines, based on an individual patient’s clinical history and surveillance data from the country or region.
The analysis for the three PICO questions on the duration of treatment did not show any differences overall in treatment failure or relapse when comparing patients with MDR-TB with or without additional second-line drug resistance, including those with additional resistance to fluoroquinolones and injectable agents. In patients with resistance to amikacin and streptomycin, Recommendation 3.17 does not apply. The duration of treatment may need to be longer than 20 months overall in MDR/RR-TB cases with extended resistance patterns, subject to the clinical response to treatment.
Rifampicin-resistant TB
A patient (child or adult) in whom isoniazid resistance is absent needs to be treated with a recommended MDR-TB regimen – either a longer MDR-TB regimen to which isoniazid is added, or a shorter MDR-TB regimen in eligible patients (see also Treatment of drug-resistant TB using 6-month regimens). Although high-dose isoniazid is not included in Groups A–C, given the rarity of its use in contemporary longer regimens for adults with MDR/RR-TB, it may still be used in patients with confirmed susceptibility or in the presence of mutations that do not usually confer complete resistance to isoniazid (82). Highdose isoniazid was shown to be an important component in paediatric regimens in a 2016 evidence review of the WHO guidelines; based on this finding its use in adults was extrapolated (71). In this analysis, high-dose isoniazid was associated with treatment success among children with confirmed MDR-TB (aOR: 5.9, 95% confidence limits [CL]: 1.7–20.5, P=0.007).
Children and adolescents
The 2018 IPD of longer regimens comprised mainly data from adult patients, with only 181 of the 13 104 (1.4%) cases being in children and adolescents aged below 15 years. Nonetheless, WHO recommendations on longer MDR-TB regimens apply to children as well as adults. Most medicines that are used in longer regimens have been part of MDR-TB regimens for many years, in similar combinations, for both adults and children. The GDG 2021 recommended the use of bedaquiline and delamanid in children of all ages (30). Reproducing the delamanid exposure achieved with the special 25 mg tablet tested in the trial in children aged 3–5 years is expected to be challenging, given that this formulation is not bioequivalent with the 50 mg delamanid adult tablet – the only preparation available at that time (2). There are also concerns that the adult tablet may shatter if attempts are made to split it, and that its contents are exceedingly bitter and unpalatable. Further, bioavailability may be altered when the 50 mg tablet is split, crushed or dissolved. Delamanid is susceptible to oxidation and heat; therefore, retaining pill fragments for use at a time other than the time of initial administration is likely to result in the delivery of lower-than-expected active compound and unspecified oxidation by-products.
The avoidance of an injectable-containing regimen is particularly desirable in children, especially those who are very young and those with mild disease (as determined by the absence of malnutrition), serious forms of extrapulmonary disease, cavitation on chest radiography or HIV infection. Hearing loss can have a permanent effect on the acquisition of language and the ability to learn at school; therefore, if amikacin or streptomycin use is resorted to in children, regular audiometry is required.
The recommendations on treatment duration apply also to children. Given that many patients in the paediatric age group may only be clinically diagnosed or have extrapulmonary disease, it is expected that treatment duration will largely be guided by Recommendation 3.15, subject to response to treatment. Shortening the total treatment duration to less than 18 months may be considered in the case of children without extensive disease (see Definitions).
Extrapulmonary TB and TB meningitis
The WHO recommendations on longer MDR-TB regimens apply also to patients with extrapulmonary disease. Adjustments may be required, depending on the specific location of the disease. Treatment of MDR/RR-TB meningitis is best guided by DST of the infecting strain and by knowledge of the properties of TB medicines that cross the blood–brain barrier. Levofloxacin and moxifloxacin penetrate the CNS well (83), as do ethionamide or prothionamide, cycloserine or terizidone, linezolid and imipenem–cilastatin (84, 85). Seizures may be more common in children with meningitis treated with imipenem–cilastatin; thus, meropenem is preferred for meningitis cases and in children. High-dose isoniazid and pyrazinamide can also reach therapeutic levels in the cerebrospinal fluid, and they may be useful if the strains are susceptible. P-aminosalicylic acid and ethambutol do not penetrate the CNS well, and they should not be counted on as effective agents for MDR/RR-TB meningitis. Amikacin and streptomycin penetrate the CNS only in the presence of meningeal inflammation. There are few data on the CNS penetration of clofazimine, bedaquiline or delamanid (86–88). In addition, cerebrospinal fluid concentrations may not mirror concentrations in the meninges or brain.
Pregnancy
Amikacin, streptomycin, prothionamide and ethionamide are usually contraindicated during pregnancy. Because of the potential for teratogenic effects from these medications, including the injectable agents, Recommendation 3.17 is of limited relevance in this subgroup. Following the changes made in the 2018 guidelines update, these agents are expected to be used less frequently in longer regimens. Knowledge about the safety of bedaquiline and delamanid in pregnancy and breastfeeding is sparse. However, new evidence from an observational study in South Africa was presented to the GDG 2019; it included information on 58 mothers who received bedaquiline during pregnancy (61). The results of this study indicated that fetal exposure to bedaquiline in utero was associated with low birth weight⁴⁷ (45% of babies exposed to bedaquiline had a low birth weight compared with 26% of babies not exposed, P=0.034) (61). However, there were no other significant differences in infant outcomes, pregnancy outcomes or maternal treatment outcomes, including weight gain in the infants until 1 year of age (61). In such cases, it is recommended that a longer regimen be individualized to include components with a better established safety profile. The outcomes of treatment and pregnancy, including data from postpartum surveillance for congenital anomalies, should be documented to help inform future recommendations for MDR-TB treatment during pregnancy.
HIV infection
The composition of the treatment regimen for MDR-TB does not usually differ substantially for PLHIV. With careful attention, it is possible to avoid certain drug–drug interactions (e.g. bedaquiline and efavirenz; see also the HIV drug interactions website of the University of Liverpool (44)).
Patients with extensive pulmonary TB disease
The duration of treatment post culture conversion may be modified according to the patient’s response to therapy⁴⁸ (e.g. culture conversion before 2 months of treatment) and other risk factors for treatment failure or relapse. This should be considered in patients with extensive TB disease.
Patients on regimens without amikacin or streptomycin
In patients on regimens that do not contain injectable agents in the intensive phase, Recommendation 3.17 does not apply, and the length of treatment is determined by recommendations on total duration and on time after culture conversion (i.e. Recommendations 3.15 and 3.16). In the future, this situation is expected to apply to an increasing proportion of patients who are treated with oral-only regimens. If bedaquiline or other agents (e.g. linezolid or delamanid) are given only for the initial part of a regimen, this period does not equate to an “intensive phase” unless an injectable agent is used concurrently, as premised by the meta-analysis that informed Recommendation 3.17.
Implementation considerations
The implementation of MDR/RR-TB treatment on a large scale is feasible under programmatic conditions, as has been shown by the global expansion in the use of standardized and individualized MDR-TB regimens in low-, middle- and high-income countries worldwide, particularly in the past decade (6). The 2018 revision of the guidelines brought important changes to the grouping of medicines, the composition of longer MDR-TB regimens and the duration of medicine use, but it is expected that implementation of these changes will be feasible. The rapidity with which the new recommendations are applied in (or to) programmes may be influenced by a range of factors, but these should not stand in the way of increased access to life-saving treatment for patients who need it.
All of the agents recommended for use are available via the GDF, and most are also available in quality-assured, affordable generic formulations from other sources. Bedaquiline was available via a donation programme until March 2019; it is now available via the GDF, and a decrease in price has been negotiated with the manufacturer for low-resource settings. The evidence assessed during the GDG meeting in November 2019 did not allow the group to make any judgements about the efficacy or effectiveness of bedaquiline when used for longer than 6 months; however, it did allow the GDG to determine that the safety profile of bedaquiline use for longer than 6 months is becoming clearer. The group concluded that bedaquiline can be safely used in patients beyond 6 months, if decided by the programme or treating clinician, and if appropriate schedules of baseline testing and monitoring are in place. In addition, the treating clinician should be aware of the use of other potentially QT-prolonging medications in any MDR/RR-TB regimen, and the comparatively long halflife of bedaquiline, which means that bedaquiline will remain in human tissue beyond the duration of its use. The half-life of bedaquiline is about 6 months, and the half-life of the N-monodesmethyl metabolite (M2) is about 5.5 months (90).⁴⁹
Concurrent bedaquiline and delamanid use
The GDG 2019 felt that there was insufficient evidence to assess the efficacy or effectiveness of the concurrent use of bedaquiline and delamanid. However, the group concluded that the safety data assessed in 2019 suggest there are no additional safety concerns regarding the concurrent use of bedaquiline and delamanid. Therefore, bedaquiline and delamanid may be used in patients who have limited options for other treatment; that is, for patients with a small number of other effective drugs included in their regimen, probably due to an extensive drug-resistance profile or intolerance to other second-line TB medications. Appropriate schedules of safety monitoring (at baseline and throughout treatment) should be in place for these patients, including ECG and electrolyte monitoring, and clinicians should be cognizant of other medicines in the regimen that can either prolong the QT interval or cause other potential adverse events.
The 2021 WHO model list of essential medicines (91) includes all agents required for longer regimens.
Drug susceptibility testing
These guidelines stress past advice that a patient’s MDR/RR-TB strain should be tested for susceptibility to medicines planned for inclusion in the regimen, so that effectiveness can be maximized. Access to rapid diagnostic testing would help clinicians to decide whether the patient is eligible for a specific MDR/RR-TB regimen, and what agents to include in a longer MDR-TB regimen. The recommendations on regimen design need to be accompanied by continued efforts to increase access to DST for medicines for which there are reliable methods, and by the development and roll-out of DST for the newer medicines. However, potentially life-saving treatment should not be withheld until all DST results become available, and empirical treatment with a regimen that is likely to be effective may need to be started, then adjusted once DST results become available.
An important observation in the 2018 IPD meta-analysis for longer regimens is that when a DST result indicates resistance to an agent, it is better to replace that agent. This also applies to medicines for which DST or the DST method used is known to be unreliable for clinical decision-making. Although DST is important for guiding effective treatment, DST results present uncertainties for several regimen components (e.g. cycloserine, streptomycin and ethambutol). “Likelihood of effectiveness” is generally assessed in the programmatic setting on the basis of one or more of the following: confirmed susceptibility in the individual patient, confirmed susceptibility in the presumed source case, no known resistance to another drug that has cross-resistance to the medicine, rare use of the medicine in an area (possibly supported by low drug-resistance levels from surveillance activities), and no previous use of the medicine in a regimen that failed to cure that same patient. When there is uncertainty about the effectiveness of a certain agent, that agent may still be included in the regimen, but it should not be considered as one of the target number of medicines needed; clinical judgement should be used to decide whether the benefit from its inclusion outweighs any added toxicity, pill burden or other disadvantages. The design of the regimen must consider the relative benefits and harms to the individual patient, including drug–drug interactions.
Dosage and duration
The guidelines update in 2018 revised the weight-based dosage schedules for medicines used in MDR-TB regimens for both children and adults (see the WHO consolidated operational handbook on tuberculosis. Module 4: Treatment and care (8)). The update to the dosages benefited from the expertise of the GDG members, and from an extensive consultation with other specialists in different fields. It was based on the latest knowledge available about the optimal use of the medicines involved (92). Adherence to the schedules is advised as far as possible. Manipulation of tablets (e.g. splitting, crushing or dissolving in water) outside their indications is to be avoided because this may interfere with the bioavailability of the drugs.⁵⁰
It is important to prevent treatment interruption, to increase the likelihood of treatment success. One measure that can help to increase retention is supporting patient adherence, either by facilitating patient visits to health care facilities or home visits by health care staff, or by using digital technologies for daily communication (93).
Monitoring and evaluation
Patients on longer MDR-TB treatment regimens need to be monitored for response to treatment and for safety, using reasonable schedules of relevant clinical and laboratory testing (15, 65). The WHO framework for aDSM needs to be applied to patients on any type of MDR/RR-TB regimen, to ensure appropriate action and an acceptable level of monitoring for adverse events and prompt response to such events – alongside monitoring for treatment outcomes. ECG may be indicated as more regimens in the future may have two or three agents that are expected to prolong the QT interval. Audiometry and specific biochemical tests should also be made available whenever certain agents are included in the regimens. Treatment in pregnancy with postpartum surveillance for congenital anomalies will help to inform future recommendations for MDR/RR-TB treatment during pregnancy.
A separate recommendation on the use of culture and microscopy to monitor bacteriological response during treatment was made in the 2018 update of the guidelines (see Monitoring patient response to MDR/RR-TB treatment regarding PICO question 11 MDR/RR-TB, 2018). Access to DST of medicines for which there are reliable methods and the development of other methods for newer medicines (e.g. sequencing) are critical (and in the case of DST, necessary) accompaniments to the treatment recommendations in these guidelines.
Patients on longer MDR-TB treatment regimens need to be monitored for treatment response and for safety, using reasonable schedules of relevant clinical and laboratory testing (15, 65). Response to treatment and toxicity are monitored through regular history-taking, physical examination and chest radiography; special tests such as audiometry, visual acuity tests and electrocardiography; and laboratory monitoring. Using smear microscopy or culture to assess conversion of bacteriological status is an important way to assess response, and most patients are expected to have converted to a sputum-negative status within the first few months of starting treatment. Persistence of culture positivity beyond that point, or close to the expected end of the intensive phase when injectable agents are in use, should trigger a review of the regimen and performance of DST. NTPs should also aim for complete registration of patients with MDR/RR-TB, through follow-up and monitoring of treatment outcomes as part of national surveillance. Regular review of MDR/RR-TB cohort data is essential.
Frameworks for the surveillance of bacteriological status, drug resistance and assignment of outcomes have been standardized in recent years (94). In contrast, systematic monitoring of adverse events during and after the end of treatment needs to be strengthened in most NTPs, given the relative novelty of active pharmacovigilance within NTPs (64, 65). The rationale for aDSM is largely supported by the increasing use worldwide of combinations of new and repurposed medications in MDR/RR-TB treatment regimens. The toxicity of certain agents may increase with the duration of use (e.g. nerve damage with linezolid), and may limit their continued use in a patient, sometimes resulting in complete cessation of treatment. The prospective collection of accurate data for key variables at the case-based level, using an electronic register, is strongly advised in the best interests of the individual patient, and to inform revisions of local and global policies (95).
37 Imipenem–cilastatin and meropenem are administered with clavulanic acid, which is available only in formulations combined with amoxicillin. Amoxicillin–clavulanic acid is not counted as an additional effective TB agent, and should not be used without imipenem–cilastatin or meropenem.
38 Given that few trials or other studies have made head-to-head comparisons of MDR-TB medicines at different dosage regimens, it is not expected that guidance on dosage adjustment will be affected by the findings of the systematic review.
39 These countries are Armenia, Bangladesh, Belarus, the Democratic People’s Republic of Korea, Ethiopia, Georgia, Indonesia, Kazakhstan, Kenya, Lesotho, Myanmar, Pakistan and Peru.
40 203 days was chosen as a cut-off as the intermodal trough of bedaquiline use for all patients in the endTB observational study was 203 days; the cut-off was not 6 months exactly, but 203 days.
41 Pharmacokinetic study to evaluate anti-mycobacterial activity of TMC207 in combination with background regimen (BR) of multidrug resistant tuberculosis (MDR-TB) medications for treatment of children/adolescents pulmonary MDR-TB (https://clinicaltrials.gov/ct2/show/NCT02354014, accessed 21 January 2022).
42 P1108. A Phase I/II, open-label, single arm study to evaluate the pharmacokinetics, safety and tolerability of bedaquiline (BDQ) in combination with optimized individualized multidrug-resistant tuberculosis (MDR-TB) therapy in HIV-infected and HIV-uninfected infants, children and adolescents with MDR-TB disease (https://www.impaactnetwork.org/studies/p1108, accessed 21 January 2022).
43 Pharmacokinetic and safety trial to determine the appropriate dose for pediatric patients with multidrug resistant tuberculosis (https://clinicaltrials.gov/ct2/show/NCT01856634, accessed 21 January 2022).
44 A 6-month safety, efficacy, and pharmacokinetic (PK) trial of delamanid in pediatric participants with multidrug resistant tuberculosis (MDR-TB) (https://clinicaltrials.gov/ct2/show/NCT01859923, accessed 21 January 2022).
45 Personal communication, K Dooley, Johns Hopkins Medicine, November 2019 – for this statement and the rest of this paragraph.
46 In the DELIBERATE trial, a Grade 1 QT adverse event was classified as an absolute QTcF in the following situations: >480 ms and ≤500 ms and QTcF change from baseline from >0 ms to ≤30 ms OR an absolute QTcF ≤480 ms and QTcF change from baseline from >30 ms to ≤60 ms. A Grade 2 QT adverse event was classified as an absolute QTcF in the following situations: >480 ms and ≤500 ms and QTcF change from baseline from >30 ms to ≤60 ms OR an absolute QTcF ≤480 ms and QTcF change from baseline >60 ms. A Grade 3 QT adverse event was classified as an absolute QTcF in the following situation: >500 ms OR an absolute QTcF >480 ms and QTcF change from baseline >60 ms. A Grade 4 QT adverse event was a life-threatening consequence; for example, torsade des pointes or other associated serious ventricular dysrhythmia (personal communication, K Dooley, Johns Hopkins Medicine, November 2019).
47 Low birth weight was defined as less than 2500 g.
48 “Bacteriological response” refers to bacteriological conversion with no reversion; “bacteriological conversion” describes a situation in a patient with bacteriologically confirmed TB where at least two consecutive cultures (for DR-TB and drug-susceptible TB) or smears (for drug-susceptible TB only), taken on different occasions at least 7 days apart, are negative; and “bacteriological reversion” describes a situation where at least two consecutive cultures (for DR-TB and drug-susceptible TB) or smears (for drug-susceptible TB only), taken on different occasions at least 7 days apart, are positive either after the bacteriological conversion or in patients without bacteriological confirmation of TB. (89)
49 This is the mean terminal half-life of bedaquiline and the M2 metabolite; this longer terminal elimination phase probably reflects the slow release of bedaquiline and M2 from peripheral tissues (90).
50 This is particularly problematic with the delamanid tablet, the contents of which are most unpalatable (see summaries of unpublished data for the 2018 guidelines update in Annex 6).

 Feedback
Feedback