Book traversal links for 1266
Recommendations 4.1–4.2 Treatment of Hr-TB

Justification and evidence
The recommendations in this section address one PICO question:
PICO question (Hr-TB, 2018): In patients with isoniazid-resistant TB (other than MDR-TB), which treatment regimen composition and duration, when compared with 6 months or more of rifampicin–pyrazinamide–ethambutol, leads to a higher likelihood of success with least possible risk of harm?
Treatment with rifampicin, ethambutol and pyrazinamide – with or without isoniazid – has been used for the treatment of patients with rifampicin-susceptible, isoniazid-resistant TB (Hr-TB) (96–98). The evidence reviewed for this guideline compared treatment regimens with isoniazid, rifampicin, ethambutol, pyrazinamide ((H)REZ)⁵¹ of different durations (e.g. 6-month regimens versus longer duration ones). Additionally, the review of evidence focused on determining whether treatment outcomes in Hr-TB patients receiving (H)REZ treatment regimens of variable duration could be improved with the addition of a fluoroquinolone or streptomycin.
The evidence used to determine the composition and duration of regimens relied primarily on an analysis of IPD that comprised 33 databases with an analysable population of 5418 Hr-TB patients. All data used to develop these recommendations were derived from observational studies conducted in various settings (33% in Europe, 31% in the Americas, 26% in Asia and 6% in Africa) (99). ⁵² In the IPD analysed, patient treatment regimens contained rifampicin, ethambutol, pyrazinamide, streptomycin, isoniazid and fluoroquinolones; thus, recommendations could be made only for regimens containing these anti-TB agents. Based on an assessment of the certainty of the evidence, carried out using predefined criteria, the certainty of the evidence was rated as very low.
Duration of (H)REZ
The analysis comparing (H)REZ treatment regimens for 6 months (6(H)REZ) and more than 6 months (>6(H)REZ) demonstrated that a 6(H)REZ regimen had a higher likelihood of treatment success than a >6(H)REZ regimen. Further analyses determined that there was no statistically significant difference in the treatment outcomes of patients receiving regimens of 6-month REZ (6REZ) and those receiving more than 6 months REZ (>6REZ). Data on intermittent dosing of the 6(H)REZ and >6(H)REZ regimens were not included; hence, no inferences could be drawn about the use of alternating versus daily regimens. The effect of length of pyrazinamide use in the (H)REZ regimen was assessed, to investigate whether the use of this medicine could be minimized to the shortest possible duration. The reduction in treatment with pyrazinamide to less than 3 months was associated with a worse treatment outcome, even with the addition of streptomycin (aOR: 0.4, 95% CL: 0.2–0.7). In 118 patients on fluoroquinolone-containing regimens who received pyrazinamide for less than 4 months, the odds of treatment success were higher than in those who received a 6(H)REZ regimen, although the difference was not statistically significant.
Duration of levofloxacin use
In a subsample of 241 patients on an (H)REZ plus fluoroquinolone regimen, the median duration of fluoroquinolone use was 6.1 months (IQR: 3.5, 8.4), and for REZ it was 9 months (IQR: 7, 11). Hence, in the observational studies that informed the IPD, it seems that treatment length was based on the completion of 6 months of treatment with fluoroquinolone.
Acquisition of drug resistance
The analysis suggested that amplification of resistance to rifampicin was lower in patients receiving the 6(H)REZ regimen (0.6%) than in those receiving >6(H)REZ (4.3%). This observation could be due to the selection and allocation of patients into specific regimens; for instance, the number of patients with extensive disease was slightly larger in those receiving >6(H)REZ. However, overall, the number of observations for each comparison was small and the effect was not statistically significant (aOR, 0.2, 95% CL: 0.02–1.70).
Adverse events
Data on adverse events were not evaluated owing to a lack of standardization (dissimilar reporting). The GDG also considered two reports containing data from patients from the United States of America (USA) in whom a detailed assessment of adverse events suggested a risk of excess hepatotoxicity with the 6(H)REZ combination (100). Drug-induced hepatotoxicity is not uncommon with anti-TB drugs. It has also been reported in individuals receiving rifampicin and pyrazinamide for 2 months for the treatment of TB infection – in such individuals, a much higher occurrence of hepatotoxicity has been observed than in those receiving only isoniazid preventive therapy (101). It is not known whether the risk of hepatotoxicity differs between 6REZ and 6HREZ.
Addition of a fluoroquinolone
In patients with Hr-TB, treatment success rates were higher when fluoroquinolones were added to (H)REZ regimens than when patients were treated with 6(H)REZ or >6(H)REZ, without the addition of fluoroquinolones (aOR: 2.8, 95% CL: 1.1–7.3). With the addition of fluoroquinolones in patients receiving (H)REZ, the number of deaths was reduced (aOR: 0.4, 95% CL: 0.2–1.1). Acquisition of additional resistance with progression to MDR-TB was also reduced when fluoroquinolones were added to a ≥6(H)REZ regimen (aOR: 0.10, 95% CL: 0.01–1.2), albeit with small absolute numbers; 0.5% (1/221) of patients on ≥6(H)REZ plus fluoroquinolones acquired resistance to rifampicin compared with 3.8% (44/1160) of patients who did not receive fluoroquinolones. Residual confounding could have increased this observed effect. The directness of the evidence was therefore downgraded because it was unclear whether fluoroquinolones were used at the beginning of treatment or only once DST results were available (in the second month or later).
Addition of streptomycin
The analysis showed that the addition of streptomycin (up to 3 months) to an (H)REZ regimen with less than 4 months of pyrazinamide decreased the likelihood of treatment success (aOR: 0.4, 95% CL: 0.2–0.7), an effect that may in part be due to confounding. The addition of streptomycin did not significantly reduce mortality (see Annex 6). There were no data on the use of other injectable agents (i.e., kanamycin, amikacin, and capreomycin) for the treatment of Hr-TB.
Treatment outcomes
When analysing the overall treatment outcomes for each one of the regimens assessed for this review, other limitations related to the characteristics of patients included in these studies were evident and could not be controlled for. Those limitations were patient selection, and allocation to treatment with specific regimens and their relationship with disease severity. Outcomes appeared to be worse in patients with cavitary disease, persistence of sputum smear positivity and previous history of TB treatment, who received a 6(H)REZ or >6(H)REZ regimen with an additional 3 months of pyrazinamide and 1–3 months of streptomycin (see Hr-TB, 2018 in Annex 5). However, the limited number of observations made it difficult to draw definitive conclusions based on the severity of TB disease or the effect of other comorbidities on this regimen.
In formulating the recommendations, the GDG assessed the overall balance between benefits and harms of an (H)REZ–levofloxacin regimen; they also considered values and preferences (paying special attention to considerations of equity, acceptability and feasibility), in addition to clinical outcomes and the potential risks of increasing toxicities (see Annex 5 for details). The conclusions of the GDG were that a regimen composed of 6 months of REZ plus fluoroquinolones was associated with higher treatment success rates (with or without the addition of isoniazid). The difference between the 6(H)REZ and >6(H)REZ regimens was modest, slightly favouring the 6-month regimen (not statistically significant). The GDG acknowledged that it was not possible to control for all possible confounding by indication when comparing the 6(H)REZ and >6(H)REZ regimens. As an example, although data on the extent of disease were not systematically captured for all patients, it is possible that a larger number of cases with extensive disease received >6(H)REZ regimens, resulting in poor outcomes for this group of patients (given the extent of disease) and possibly favouring the 6(H)REZ regimen.
The GDG acknowledged the safety implications of (H)REZ–levofloxacin, particularly the hepatotoxicity associated with prolonged use of pyrazinamide-containing multidrug regimens. However, reducing the duration of the treatment with pyrazinamide to 3 months or less was associated with worse treatment outcomes, at least in Hr-TB regimens without a fluoroquinolone. Furthermore, the use of streptomycin in these regimens was associated with no significant added benefit. The use of streptomycin and other injectable agents has also been associated with increased serious adverse events (102–104). On this basis, the GDG agreed that current data supported the use of the (H)REZ–levofloxacin regimen without streptomycin or any other injectable agent in Hr-TB cases, unless there is a compelling reason to use these agents (e.g. certain forms of polydrug resistance).
The GDG also noted that patients were likely to place a high value on a 6-month regimen, the likelihood of a relapse-free successful outcome and, especially, the implementation of a regimen without the use of injectable agents. GDG members agreed that the use of the 6(H)REZ regimen would probably increase health equity, given that the cost of the components is relatively low (compared with the recommended regimens for MDR/RR-TB) and the increased probability of cure in a substantial number of patients. In addition, the exclusion of streptomycin and other injectable agents reduces potential barriers to regimen administration.
Although patient costs were not factored into the analysis, the GDG agreed that improving diagnostic capacity to detect isoniazid resistance would be beneficial. A modelling analysis performed for the 2011 update of the WHO Guidelines for the programmatic management of drug-resistant tuberculosis (13) estimated that the best strategy for averting deaths and preventing acquired MDR-TB was to undertake DST in all patients before treatment, using a rapid test that detects resistance to isoniazid and rifampicin (105). The modelling work also showed that rapid testing for resistance to both isoniazid and rifampicin at the time of diagnosis was the most cost-effective testing strategy for any patient group or setting, even at very low levels of resistance among TB patients (MDR-TB in >1% and isoniazid resistance [other than MDR-TB] in >2%).
In general, the GDG considered that the use of the 6(H)REZ–levofloxacin regimen would be feasible in most DR-TB treatment settings, and that the use of a regimen based on medicines that are administered orally may increase feasibility. Altogether, based on present evidence, when discussing the balance between benefits and harms, preferences and values for patients and other end-users, the GDG reached overall agreement on the beneficial effect that the Hr-TB regimen may have, if used in conformity with these policy recommendations. Although there was no clear evidence to suggest that the addition of isoniazid to this regimen would be beneficial, the four-drug (H)REZ fixed-dose combination (FDC) may be more convenient for the patient and the health service because it removes the need to use single drugs.
Consistent with the overall framework for the management and care of patients diagnosed with DR-TB, careful selection of patients is a fundamental principle. Ahead of starting the (H)REZ–levofloxacin regimen, it is essential that resistance to rifampicin be excluded, using WHO-recommended genotypic or phenotypic methods (41, 106). Ideally, resistance to fluoroquinolones (and, if possible, to pyrazinamide) should be similarly excluded before treatment, to help avert the acquisition of additional drug resistance (see Implementation considerations).
Empirical treatment of Hr-TB is generally not advised. In cases where a diagnosis of Hr-TB is strongly presumed (e.g. close contacts of Hr-TB cases with active TB but without laboratory confirmation of Hr-TB), (H)REZ–levofloxacin may be introduced pending laboratory confirmation of isoniazid resistance, provided that rifampicin resistance has been reliably excluded. Should DST results eventually indicate susceptibility to isoniazid, levofloxacin is stopped, and the patient completes a 2HREZ/4HR regimen (i.e. 2 months of HREZ followed by 4 months of HR). For patients in whom Hr-TB is detected after the start of treatment with the 2HREZ/4HR regimen, the (H)REZ component drugs are continued (or pyrazinamide and ethambutol are reintroduced) and levofloxacin added, once rifampicin resistance has been excluded.
The duration of an (H)REZ–levofloxacin regimen is usually determined by the need to complete 6 months of a levofloxacin-containing regimen. Thus, in cases where the diagnosis of Hr-TB is made after first-line TB treatment has already been initiated, the patient may receive more than 6 months of (H)REZ by the end of treatment. When the confirmation of isoniazid resistance arrives late into treatment with a 2HREZ/4HR regimen (e.g. 5 months after start during the continuation phase), the clinician would need to decide, based on an assessment of the patient’s condition, whether a 6-month course of (H)REZ–levofloxacin needs to be started at that point or not.
The addition of levofloxacin to (H)REZ is recommended in all patients with Hr-TB, with the exception of the following situations: resistance to rifampicin cannot be excluded; known or suspected resistance to levofloxacin; known intolerance to fluoroquinolones; known or suspected risk for prolonged QT interval; and pregnancy or during breastfeeding (not an absolute contraindication). In a patient with Hr-TB in whom a fluoroquinolone cannot be used, the patient may still be treated with 6(H)REZ.
When additional resistance (especially to pyrazinamide) is suspected or confirmed, appropriate treatment regimens will have to be designed individually. The data reviewed for this guideline could not provide separate evidence-based recommendations for such cases.
Where possible, isoniazid resistance testing should also include information on the specific mutations associated with resistance to isoniazid (katG or inhA). In addition, knowledge about overall host acetylator⁵³ status at country or regional level will be useful, given that these may have implications for regimen design (107).
Automated, cartridge-based and high-throughput diagnostic platforms are available (as an alternative to LPA) and countries have the capacity to use them. These platforms can, simultaneously or in separate tests, detect TB, and resistance to rifampicin, fluoroquinolones and isoniazid.
Subgroup considerations
Children
In the IPD review, only 2% of Hr-TB patients were children; thus, a separate estimate of effect for paediatric patients was not possible. However, there is no reason why the results and recommendations cannot be extrapolated from adults to children, considering that the regimen components have been standard paediatric TB medicines for many years.
Patients with extensive disease
Although the IPD analysis did not provide evidence for duration of treatment extension, the prolongation of the 6(H)REZ–levofloxacin regimen to more than 6 months could be considered on an individual basis for patients with extensive disease (108). Prolongation of treatment may increase the risk of adverse events in some cases (see Implementation considerations).
People living with HIV
The effect of longer duration TB treatment among PLHIV with and without ART has been studied among patients with drug-susceptible TB (109). In these cases, relapse has been reported to be 2.4 times higher in PLHIV who were not on ART and who received 6 months of treatment than in patients in whom treatment was prolonged (up to 9 months). In patients with drug-susceptible TB initiated on ART, no significant benefit from prolonging rifampicin-containing regimens for over 6 months has been observed (93). In the current analysis, only a limited number of patients received ART; nonetheless, in TB patients with HIV coinfection, the first priority is to ensure that they are started on ART within 8 weeks of TB treatment initiation (regardless of CD4 count), in accordance with WHO guidelines (110). The 6(H)REZ–levofloxacin regimen is therefore recommended in PLHIV.
Extrapulmonary disease
No data were available for patients with exclusive extrapulmonary Hr-TB. The regimen composition proposed is likely to be effective even in these patients. However, the treatment of patients with extrapulmonary TB should be designed in close consultation with appropriate specialists (e.g. infectious disease physicians and neurologists), to decide upon individual variations in treatment duration and supportive care, as needed.
Implementation considerations
Case scenarios
Implementing these recommendations requires the (H)REZ–levofloxacin regimen to be administered only in patients in whom resistance to isoniazid has been confirmed and resistance to rifampicin has been excluded. Preferably, testing for resistance to fluoroquinolones (and, if possible, to pyrazinamide) is also done before starting treatment. It is envisaged that the treatment regimen for Hr-TB will apply in the following situations:
- Hr-TB and rifampicin susceptibility are confirmed before TB treatment is started. Treatment with (H)REZ–levofloxacin is started immediately. If the diagnosis is strongly presumed (e.g. close contacts of a confirmed Hr-TB source case) but results of DST are still pending, the regimen may be introduced. If the DST results taken at the start eventually show susceptibility to isoniazid, then levofloxacin is stopped, and the patient continues treatment to complete a 2HREZ/4HR regimen.
- Hr-TB is confirmed after the start of treatment with the 2HREZ/4HR regimen. This includes patients who had undiagnosed isoniazid resistance initially or who developed isoniazid resistance later while on treatment with a first-line regimen. In such cases, rapid molecular testing for rifampicin resistance must be done (or repeated). Once rifampicin resistance has been excluded, a full 6-month course of (H)REZ–levofloxacin is given. The duration is driven by the need to give levofloxacin for 6 months, which usually implies that the companion first-line medicines are taken for longer than this.
If rifampicin resistance is detected, the patient needs to be started on a recommended MDR-TB treatment regimen, as described in other sections of these guidelines.
Diagnostic capabilities
The overall aim of TB treatment is to achieve cure without relapse in all patients, interrupting M. tuberculosis transmission and preventing the acquisition (or amplification) of additional drug resistance. Globally, Hr-TB is more prevalent than MDR-TB. Thus, all countries need to move towards universal testing of both isoniazid and rifampicin resistance at the start of TB treatment, and to ensuring careful selection of patients eligible for the (H)REZ–levofloxacin regimen.⁵⁴ The minimum diagnostic capacity to appropriately implement these recommendations is rapid molecular testing for rifampicin resistance before the start of treatment with the Hr-TB regimen and, preferably, the ruling out of fluoroquinolone resistance using WHO-recommended tests.
Rapid molecular tests such as Xpert MTB/RIF, Xpert MTB/XDR and LPAs are preferred, to guide patient selection for the (H)REZ–levofloxacin regimen (41, 111).
Surveillance of DR-TB indicates that fluoroquinolone resistance among patients with rifampicinsusceptible TB is generally low worldwide (112). However, national data on the prevalence of fluoroquinolone resistance – including targeted or whole-genome sequencing to detect specific mutations associated with resistance to fluoroquinolones (62) – could help to guide testing policies when countries implement the Hr-TB treatment recommendations.
When additional resistance (e.g. to both fluoroquinolones and pyrazinamide) is suspected or confirmed, treatment regimens that include other second-line TB medicines may have to be designed individually. The current review could not provide further evidence on effective regimens in patients with polyresistant disease.
Support and close monitoring of patients are needed to maximize treatment adherence and enable early detection of patients who are not responding to treatment (e.g. those with persistent sputum culture or smear positivity). In the presence of non-response to treatment, DST for rifampicin and the fluoroquinolones should be repeated, preferably with Xpert MTB/XDR or LPA. Documented acquisition of resistance to rifampicin or a fluoroquinolone while on the Hr-TB treatment regimen should alert the clinician to the need to review the entire clinical and microbiological status of the patient, and change the regimen where necessary.
Levofloxacin is proposed as the fluoroquinolone of first choice in the Hr-TB treatment regimen for several reasons. First, the safety profile of this medicine is better characterized than that of other fluoroquinolones, and levofloxacin was the fluoroquinolone most frequently used in the studies reviewed for this guidance. Second, in comparison to moxifloxacin, levofloxacin has fewer known drug interactions with other medications. For example, although both plasma peak concentration and exposure to moxifloxacin decrease significantly when the drug is combined with rifampicin (113), the same effect has not been reported for levofloxacin, possibly because levofloxacin undergoes limited metabolism in humans and is excreted unchanged in the urine (114). Third, although levofloxacin may interfere with lamivudine clearance, in contrast to moxifloxacin, there are no contraindications for its use with other antiretroviral agents (44).
The addition of levofloxacin to (H)REZ is recommended in patients with Hr-TB, with the exception of the following situations:
- resistance to rifampicin cannot be excluded (i.e. unknown susceptibility to rifampicin, or indeterminate or error results on Xpert MTB/XDR);
- known or suspected resistance to levofloxacin;
- known intolerance to fluoroquinolones;
- known or suspected risk for prolonged QT interval; and⁵⁵
- if possible, in pregnancy or during breastfeeding (not an absolute contraindication).
Sometimes, the confirmation of isoniazid resistance arrives late (e.g. 5 months into a 2HREZ/4HR regimen). In such cases, a decision to start 6 months of (H)REZ–levofloxacin depends on the patient’s clinical condition and microbiological status.
If levofloxacin cannot be used because of toxicity or resistance, the patient may be given 6(H)REZ as an alternative. Based on the results of the evidence review for these guidelines, replacement of levofloxacin with an injectable agent is NOT advised. The evidence review could not inform on the effect of other second-line TB medicines on treatment effectiveness.
Addition of isoniazid
There was no clear evidence that the addition of isoniazid affects patients (i.e. adding benefit or harm). For patient convenience and ease of administration, the four-drug HREZ FDCs⁵⁶ may be used to deliver the Hr-TB treatment regimen alongside levofloxacin.
The use of high-dose isoniazid (10–15 mg/kg per day in adults) was not evaluated in this review owing to insufficient data. However, the GDG discussed the effect of increasing isoniazid dosing beyond that provided in weight-banded FDCs, depending on the type of molecular mutations identified. In vitro evidence suggests that when specific inhA mutations are detected (and when katG mutations are absent), increasing the dose of isoniazid is likely to be effective; thus, additional isoniazid up to a maximum dose of 15 mg/kg per day could be considered. In the case of katG mutations, which usually confer a higher level resistance, the use of isoniazid even at a higher dose is less likely to be effective (115).⁵⁷
Dosage
Although the IPD analysis did not provide evidence to address the frequency of dosing, it is best to avoid intermittent or divided dosing of the 6(H)REZ–levofloxacin regimen (27, 93, 116). In the absence of full information about optimal drug doses, a weight-band dosing scheme for levofloxacin is recommended.⁵⁸
Drug–drug interactions
Levofloxacin may interfere with lamivudine clearance (increasing the levels of lamivudine) but it is not contraindicated with other antiretroviral agents, and no drug dosing adjustments are needed (44). Co-administration of levofloxacin with oral divalent cation-containing compounds (e.g. antacids) may impair its absorption and should be avoided (15). Restriction of concomitant use of milk products is not necessary.
Treatment prolongation beyond 6 months
Prolonging of treatment beyond 6 months may be considered for patients with extensive disease or in those slow to convert to smear or culture negative. In the latter, acquisition of additional resistance to rifampicin must be ruled out, as must resistance to fluoroquinolones and pyrazinamide, if possible. Such patients require careful monitoring and follow-up.
Cost
A cost–effectiveness analysis was not performed for this review. Table 4.1 presents approximate prices for a full course of medicines with the different regimens in adults, based on the cost of products available from the GDF (50). Use of FDCs, even for part of the regimen, reduces costs. Medicines needed for a 6HREZ–levofloxacin regimen cost about three times as much as a 2HREZ/4HR regimen when using the HREZ FDC. The treatment of Hr-TB according to these guidelines is not expected to significantly increase operational costs.
Table 4.1. Illustrative costs of regimens used to treat Hr-TB compared with the 6-month first-line TB regimen
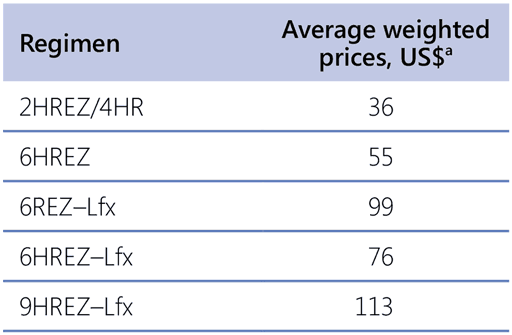
FDC: fixed-dose combination; HR: isoniazid and rifampicin; HREZ: isoniazid, rifampicin, ethambutol and pyrazinamide; Hr-TB: rifampicinsusceptible, isoniazid-resistant; Lfx: levofloxacin; REZ: rifampicin, ethambutol and pyrazinamide; TB: tuberculosis.
a Prices are as of 15 March 2020 for a 60 kg adult, and they reflect the use of FDCs whenever possible. Average weighted prices are based on prospective market share allocation and are indicative only. For budgeting purposes, it is recommended to use the budgeting prices from the Stop TB Partnership (50).
Source: Stop TB Partnership (2020) (50).
Adherence
The IPD analysis contained limited data on the treatment adherence strategies used, such as directly observed treatment and self-administered therapy (SAT). Improved treatment success rates appeared to be associated with increased patient support, including medication adherence support (e.g. by means of digital technologies) or other means, as recommended by WHO (93). In contrast to regimens for drug-susceptible TB and MDR-TB, the recommended Hr-TB treatment regimen does not have an intensive phase and a continuation phase, simplifying the delivery and monitoring of treatment.
Monitoring and evaluation
Patients who receive the (H)REZ–levofloxacin regimen need to be monitored during treatment, using schedules of clinical and laboratory testing. The definitions to use when assigning outcomes are the same as those used for drug-susceptible TB (94). Signs of non-response or treatment failure should be followed up with DST for rifampicin resistance and, if possible, for fluoroquinolones and pyrazinamide. To limit the risk of acquisition of additional resistance, the addition of single TB medicines should be avoided in patients who remain smear positive or culture positive after month 2 of treatment, those who do not show a favourable clinical response and those without recent DST results.
As with any other TB medicine and regimen, safety precautions are required to ensure the rapid identification and proper management of any serious adverse event. Close clinical monitoring is essential for all patients receiving this regimen, particularly liver function tests, given the hepatotoxic potential of prolonged pyrazinamide use. If possible, all patients should be tested each month for levels of AST (also known as serum glutamic oxaloacetic transaminase, SGOT). If resources are not available to monitor all patients on the Hr-TB treatment regimen, monthly monitoring of patients at high risk (e.g. patients coinfected with viral hepatitis or with a history of heavy alcohol use) is strongly advised. Additionally, to prevent and manage the potential toxic effects of ethambutol in children (e.g. retrobulbar neuritis), it is necessary to adhere to the correct doses recommended for paediatric populations. Early signs of ethambutol toxicity can be tested in older children through red–green colour discrimination. Monitoring for retrobulbar neuritis can be undertaken early when appropriate (117).
51 “(H)” indicates that isoniazid is optional.
52 The number of patients highlighted in this section refers to the sample size of each study. However, the analysable sample size was later modified, depending on the availability of IPD for each analysable outcome (success and mortality).
53 Decreased efficacy and toxicity of isoniazid have been related to its increased metabolism (acetylation) in certain individuals, as determined by mutations in the N-acetyltransferase type 2 (NAT2) gene.
54 The association between previous TB treatment history and Hr-TB is less strong than the association in MDR-TB. As a result, previous TB treatment is less reliable as a proxy for Hr-TB and a laboratory diagnosis is therefore important.
55 Baseline-corrected QT. Prolongation of the QT interval and isolated cases of torsades de pointes have been reported. Avoid use in patients with known prolongation, those with hypokalaemia, and with other drugs that prolong the QT interval.
56 Although most countries currently procure the four-drug FDC via the Stop TB Partnership’s GDF, in settings where only the three-drug combination FDC (i.e. HRZ) is available, ethambutol has to be administered separately.
57 An isolated katG or inhA mutation can correspond to variable MIC levels. This implies that inhA mutations do not always indicate lowlevel isoniazid resistance, and that katG mutations are not necessarily correlated with high-level isoniazid resistance. However, the presence of both mutations is usually an indication of high-level resistance (115).
58 Studies included in this IPD analysis involved the use of regimens containing levofloxacin (usually at a dose of 750–1000 mg/day), moxifloxacin (400 mg/day) or gatifloxacin (400 mg/day), as well as early-generation fluoroquinolones (ciprofloxacin and ofloxacin), which are no longer recommended for the treatment of DR-TB. Gatifloxacin is currently unavailable in quality-assured formulations, and ciprofloxacin and ofloxacin are no longer recommended for use in DR-TB care.

 Feedback
Feedback