Enlaces transversales de Book para PLATAFORMA DE INTERCAMBIO DE CONOCIMIENTOS SOBRE LA TB DE LA OMS
3.2.1 Background
WHO defines advanced HIV disease for adults and adolescents (and children five years and older) as having a CD4 cell count of less than 200 cells/mm3 or WHO clinical stage 3 or 4 disease (13). All children younger than five years living with HIV are considered to have advanced HIV disease. People presenting with advanced HIV disease are at high risk of death, even after starting ART, with the risk increasing with decreasing CD4 cell count, especially with CD4 cell count < 100 cells/ mm3 (98-101). Advanced HIV disease is also associated with increased healthcare costs (102), increased risk of opportunistic infections, immune reconstitution inflammatory syndrome (IRIS), incomplete immune reconstitution, higher viral reservoirs, higher inflammation, increased risk of HIV-related and non-HIV-related comorbidities, use of more healthcare services and more frequent monitoring needs (102).
To address the leading causes of morbidity and mortality among people with advanced HIV disease, WHO recommends that a package of interventions including screening, treatment and/or prophylaxis for major opportunistic infections, rapid ART initiation, and intensified adherence support interventions, be offered to everyone (all populations and age groups) living with HIV presenting with advanced HIV disease.
3.2.2 Summary of evidence and rationale
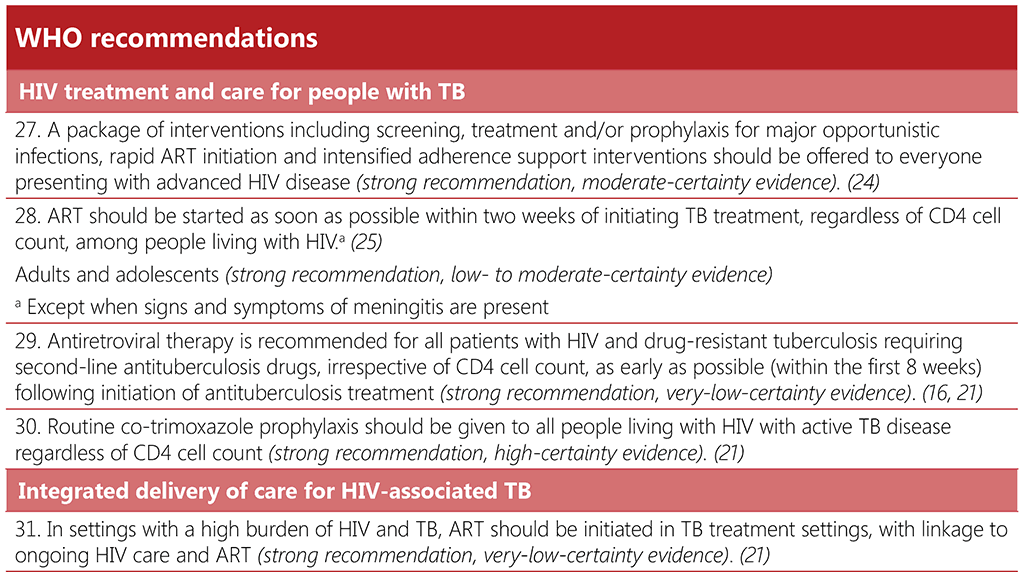
Recommendation 27: Package of interventions for people with advanced HIV disease
Tuberculosis is a marker for advanced HIV disease and is well recognized as a leading cause of morbidity and mortality among people living with HIV. People with HIV and TB have a higher risk of mortality compared with people with TB who do not have HIV. Other causes of mortality among adults with advanced HIV disease globally include severe bacterial infections, cryptococcal disease, histoplasmosis, toxoplasmosis and Pneumocystis jirovecii pneumonia. Addressing these other comorbidities as part of an integrated package of care for people with HIV-associated TB can help to reduce mortality. TB and HIV programmes are therefore encouraged to work together to expand access to an integrated package of care for advanced HIV disease among people with TB.
This recommendation is based on two RCTs: REMSTART (103) and REALITY (104). REMSTART was conducted in the United Republic of Tanzania and Zambia, and randomized 1999 ART-naïve adults with HIV with CD4 count <200 cells/mm3 to either standard care or standard care plus enhanced clinic-based care with serum cryptococcal antigen (CrAg) screening, pre-emptive antifungal treatment for those who screened positive for CrAg, as well as additional community support (comprising a weekly home or community visit by trained and paid lay workers who delivered ART, provided adherence support and monitored participants for signs and symptoms of drug toxicity or new symptoms). The intervention group had 28% fewer people dying: mortality was 13% in the intervention group versus 18% in the group receiving standard care (103).
The REALITY study enrolled 1805 people living with HIV with CD4 counts < 100 cells/mm3 in Kenya, Malawi, Uganda and Zimbabwe (104). Participants were mainly adults (72 were 5–17 years old). All underwent screening for TB disease at enrolment and were then randomized to the standard of care (co-trimoxazole) according to national guidelines or an enhanced prophylaxis package: 12 weeks of fluconazole (100 mg once daily), 12 weeks of a fixed-dose combination of co-trimoxazole (800 + 160 mg) + isoniazid (300 mg) + pyridoxine (25 mg) as a scored once-daily tablet, five days of 500 mg of azithromycin once daily and a single dose of 400 mg of albendazole. All drugs were started simultaneously, and ART was offered on the same day as the prophylaxis package. The enhanced prophylaxis package at the time of ART initiation reduced mortality by 27% (from 12.2% to 8.9%) over 24 weeks. Mortality from Cryptococcus species declined considerably, from 1.5% to 0.4%, and mortality from unascertained causes (most people died at home) declined from 6.0% to 3.8%. TB incidence was reduced by 28%, cryptococcal disease by 62% and hospitalization by 17% in the enhanced prophylaxis group versus the standard-of-care group. Most of the deaths in this study occurred within the first three weeks, highlighting the value of early prophylaxis for people with advanced disease (104).
Recommendations 28–29: Timing of ART initiation for HIV-associated TB
Early initiation of ART for people living with HIV-associated TB is critical in reducing morbidity and mortality and preventing HIV transmission. HIV and TB programmes should ensure that people with TB who also have HIV are offered ART as early as possible, preferably within integrated services or within TB facilities (10). In 2010, WHO recommended that ART be started as soon as possible within eight weeks of initiating TB treatment, and in 2012, WHO recommended to initiate ART within two weeks among those with CD4 count ≤ 50 cells/mm3. Since 2021, WHO has recommended that ART should be started as soon as possible within two weeks of initiating TB treatment, regardless of CD4 cell count for people with drug-susceptible TB and in whom TB meningitis is excluded (21).
Timing of ART initiation for people living with HIV diagnosed with drug-susceptible TB
This recommendation was informed by a systematic review and meta-analysis which identified nine trials that compared earlier ART to later ART initiation in people with HIV and TB. Four studies provided information on ART initiation within two weeks of starting TB treatment and between two and eight weeks from the commencement of TB treatment (105-108).
Moderate-certainty evidence indicates that mortality may be similar with ART initiated within two weeks of TB treatment versus ART initiated between two and eight weeks from the start of TB treatment (risk difference = –0.01; 95% CI: –0.06 to 0.04), which can be interpreted as one less death per 100 people, ranging from 6 fewer deaths to 4 more deaths per 100 people.
In a sub-analysis of people with a CD4 cell count less than or equal to 50 cells/mm3, low- certainty evidence indicated that mortality may not differ (3 fewer deaths per 100 people, 95% CI: from 10 fewer to 4 more, per 100 people) with ART initiated within two weeks of TB treatment versus between two weeks and eight weeks. Among the subgroup with CD4 cell count greater than 50 cells/mm3, low-certainty evidence indicated that mortality may be similar with earlier ART initiation (2 fewer deaths per 100, 95% CI: from 7 fewer to 4 more deaths per 100 people) with ART initiated within two weeks of TB treatment versus between two weeks and eight weeks.
Low-certainty evidence indicated that AIDS-defining events (for all CD4 cell counts) may be similar with ART initiation within two weeks of TB treatment initiation versus ART initiation between two and eight weeks from TB treatment initiation (2 fewer AIDS-defining events per 100 people, 95% CI: 6 fewer to 3 more per 100 people). Among people living with HIV with any CD4 cell count, low-certainty evidence indicated that viral load suppression also may not differ between people initiating ART within two weeks versus those initiating ART between two and eight weeks from TB treatment initiation (1 person with viral load suppression less per 100 people, 95% CI: from 3 fewer to 6 more per 100 people).
Very low-certainty evidence indicated that the incidence of IRIS events may be increased among people offered ART initiation within two weeks from TB treatment initiation versus those initiating ART between two and eight weeks from TB treatment initiation (7 more events per 100 people, 95% CI: 3 fewer events to 17 more events per 100 people). However, mortality related to IRIS was uncommon.
Therefore, based on the public health approach, and after weighing up the evidence on the potential harms of mortality, AIDS-defining events and IRIS against the benefits of early start of ART among all people living with HIV, WHO now recommends that people with HIV and drug-susceptible TB should start ART within two weeks of TB treatment initiation, regardless of CD4 cell-count.
Among people living with HIV with TB meningitis, immediate ART is associated with more severe adverse events compared with initiating ART two months after the start of TB treatment. The expert opinion of the GDG was that ART should be delayed by at least four weeks (and initiated within eight weeks) after TB treatment is initiated for TB meningitis, due to safety concerns.
Whilst there have been concerns about the possible increased risk of IRIS in DTG-based regimens, the INSPIRING trial (109) reported that the incidence of IRIS was similar between the DTG and efavirenz arms (in this small trial of safety and efficacy of rifampicin-based TB treatment and ART initiated within eight weeks). These findings were consistent with the 2019 network meta-analysis undertaken to inform the 2019 WHO ARV drug guidelines update, with the safety of DTG examined among people with both TB and HIV. No deaths were reported in either arm (DTG versus efavirenz), and there were fewer severe adverse events in the DTG arm (odds ratio: 0.61, 95% CI: 0.17–2.24), with low-certainty evidence (110). The REALITY trial among people with advanced HIV disease included raltegravir as an additional option and also did not find any increased incidence of IRIS (104). However, close follow-up is advisable to monitor IRIS and other clinical events requiring prompt assessment and management, especially among children and pregnant or breastfeeding women. HIV programmes and service providers should establish mechanisms for adequate monitoring, including pharmacovigilance and surveillance for drug-drug interactions.
The review did not identify any studies that included pregnant and breastfeeding women. However, the GDG noted that earlier ART was unlikely to increase harm in this population, and the well-known and demonstrable benefits of earlier ART for both the mother’s health and the child’s health, with reduced vertical transmission of HIV, outweighed potential harm (21). Evidence was also limited regarding the timing of ART for those with drug-resistant TB and those receiving second- and third-line ART regimens.
Timing of ART initiation for people living with HIV diagnosed with drug-resistant TB
Evidence was reviewed from 10 studies (111-120) to assess treatment outcomes when ART and second-line TB drugs were used together. None of the data were from RCTs. Individual participant data were available for 217 people with drug-resistant TB in total, of whom 127 received ART. The level of evidence in individual observational studies varied from a low- to a very low-certainty.
The pooled individual participant data from longitudinal cohort studies showed a lower risk of death and a higher likelihood of cure and resolution of TB signs and symptoms in individuals using ART compared with those not using ART (low-quality evidence). There is very low-quality evidence for other outcomes that were considered critical or important for decision-making (for example, severe adverse effects from second-line drugs for DR-TB, occurrence of sputum smear or culture conversion, interactions of ART with TB drugs and default from treatment). Available data did not allow assessment for a number of other outcomes of interest, namely, avoiding the acquisition of additional drug resistance, preventing TB transmission, sustaining relapse-free cure, establishing the optimal duration of MDR-TB treatment, avoiding unnecessary MDR-TB treatment, reducing cost and improving population access to appropriate care.
Recommendation 30: Co-trimoxazole prophylaxis for people living with HIV with diagnosed TB
Co-trimoxazole is a fixed-dose combination of two broad-spectrum antimicrobial agents (sulfamethoxazole and trimethoprim) that prevents a range of secondary bacterial, fungal and protozoan infections. People living with HIV who also have TB disease should receive co-trimoxazole regardless of CD4 count. Evidence from RCTs, including areas with high levels of antibiotic resistance, has shown reduced mortality, morbidity and hospitalization with no significant increase in adverse events among people living with HIV who have smear-positive TB, regardless of their CD4 counts (121, 122). Other non-randomized and operational studies showed that co-trimoxazole preventive therapy is feasible (123, 124), safe and reduces mortality rates in people with TB (123, 125).
Recommendation 31: ART initiation in TB treatment settings and linkages to HIV care
Coordination between TB and HIV programmes to deliver comprehensive and uninterrupted care for TB and HIV is important for the individual in need of care. It can also reduce out-of-pocket costs related to travelling to multiple appointments (126). Community engagement, patient education, engagement of adherence counsellors and social workers and peer support for early recognition of adverse events and to support retention and adherence to co-treatment are also needed, as well as for continuation of ART after TB treatment completion.
A systematic review evaluating the effectiveness of delivering ART in TB treatment settings identified 19 observational studies, many of which showed increased uptake and timeliness of ART initiation. However, the data on mortality and TB treatment success were inconsistent. The same systematic review identified five observational studies evaluating the effectiveness of delivering TB treatment in HIV care settings. Two studies reported decreased mortality, and another showed comparable mortality rates. The TB treatment success rates and ART uptake were comparable across studies (44).

 Reacción
Reacción