كتاب روابط اجتياز لـ 1266
Over recent decades, considerable effort has gone into building the laboratory, clinical and programmatic capacity to prevent, detect and treat TB infection, TB disease and DR-TB. Many tools and guidance documents have been developed, including the recently consolidated and updated guidelines for the treatment of TB (8); blood-based tests for TB infection that incorporate chemiluminescent methods for result detection; urine-based biomarker point-of-care (POC) tests for TB detection among people living with HIV; low-complexity tests that can use stool samples from children to detect TB and resistance to RIF; low-complexity tests that laboratories with basic infrastructure can use to detect resistance to RIF, INH, FQs, ethionamide (ETO) and amikacin (AMK); genomic sequencing technologies that incorporate resistance interpretation analyses that consider advances in our understanding of the mechanisms of drug resistance; and consolidated model diagnostic testing algorithms and guidance for implementing testing programmes that are tailored to the purpose of testing and characteristics of the patient population (e.g. age, HIV status and risk of drug resistance).
Until 2024, the operational guidance that supported advancing WHO policy on testing for TB infection, diagnosis and drug resistance was presented separately (in operational handbooks on tests for TB infection, and on tests for TB diagnosis and drug resistance). This handbook is the first to combine and update this operational guidance into a single reference document. In addition, an increasing number of novel TB tests may be used for the purposes listed above; hence, the WHO Global Programme on Tuberculosis & Lung Health (WHO/ GTB) has adopted “class-based recommendations” that apply to TB testing products with similar characteristics and performances. In addition, WHO prequalification (PQ) has been established to assess whether each testing product within a class, and the process used to manufacture that product, meet performance and quality standards (9). This approach to TB test assessment, recommendation and PQ is expected to increase competitiveness in price, quality and services.
New TB testing products will continue to be reviewed for class determination by WHO/ GTB, by comparing their characteristics with those of the existing diagnostic classes. If the characteristics differ sufficiently to warrant a new class, the product will be assessed as “first in class” using a standardized, Pathway A, WHO/GTB guideline development process, supported by a guideline development group (GDG). If the characteristics are sufficiently similar to an existing class, the product will be characterized as “within class” and may be referred directly to PQ. If a WHO PQ process has not yet been established for a given class, then WHO/GTB will oversee an interim within-class assessment of evidence with the support of the Technical Advisory Group (TAG) on TB Diagnostics and Laboratory Strengthening. Further details on class determination and product assessments are available in the 2025 publication, WHO consolidated guidelines on tuberculosis: module 3: diagnosis (5).
In 2024 and early 2025, WHO oversaw both first-in-class and within-class assessments. Through the first-in-class assessment, two new classes of low-complexity nucleic acid amplification tests were established. Both classes include testing products previously endorsed by WHO for the detection of TB with and without RIF resistance (i.e. Xpert® MTB/RIF Ultra, Molbio Truenat® MTB Plus and MTB-RIF Dx, and TB-LAMP) (Section 2). There are also new recommendations on concurrent testing of respiratory and non-respiratory samples among adults and adolescents with HIV, children with HIV, and children without HIV or with unknown HIV status. These populations experience significant burdens of TB, increased risk of TB-associated morbidity and mortality, and challenges with sputum production; in addition, they may produce sputa with low and variable amounts of bacteria. Hence, concurrent testing may increase patient access to testing services through the included use of easy-to-collect urine and stool samples, while improving the accuracy of TB detection by using more than one sample and test.
Practical considerations for implementation of the concurrent testing recommendations are presented in Section 3 and are reflected in the revised model diagnostic algorithms and decision pathways given in Section 6.
In addition, the 2025 within-class assessment evaluated new interferon-gamma release assays (IGRAs) for the detection of TB infection, and new and updated targeted NGS solutions for the detection of DR-TB. WHO recommendations for the class of IGRAs now apply to two new tests (SD Biosensor STANDARD E TB-Feron ELISA and Diasorin/QIAGEN LIAISON QFT-PLUS CLIA), which expand the list of WHO-recommended tests for TB infection and introduce the chemiluminescent detection method for IGRA testing for the first time. The recommendations for the class of targeted NGS apply to one updated solution (Oxford Nanopore Technologies AmPORE-TB) that can now be used to detect resistance to a wider range of TB drugs. Policy statements on the performance of the new technologies are summarized in the relevant portions of Section 2, new within-class products are highlighted in the full list of TB diagnostic classes and testing technologies in Table 2.1, and the TAG meeting and evidence review reports are provided in Web Annex D.
The full list of current TB testing recommendations is presented, with policy details, in the 2025 publication, WHO consolidated guidelines on tuberculosis: module 3: diagnosis (5) and in Table 1.1.
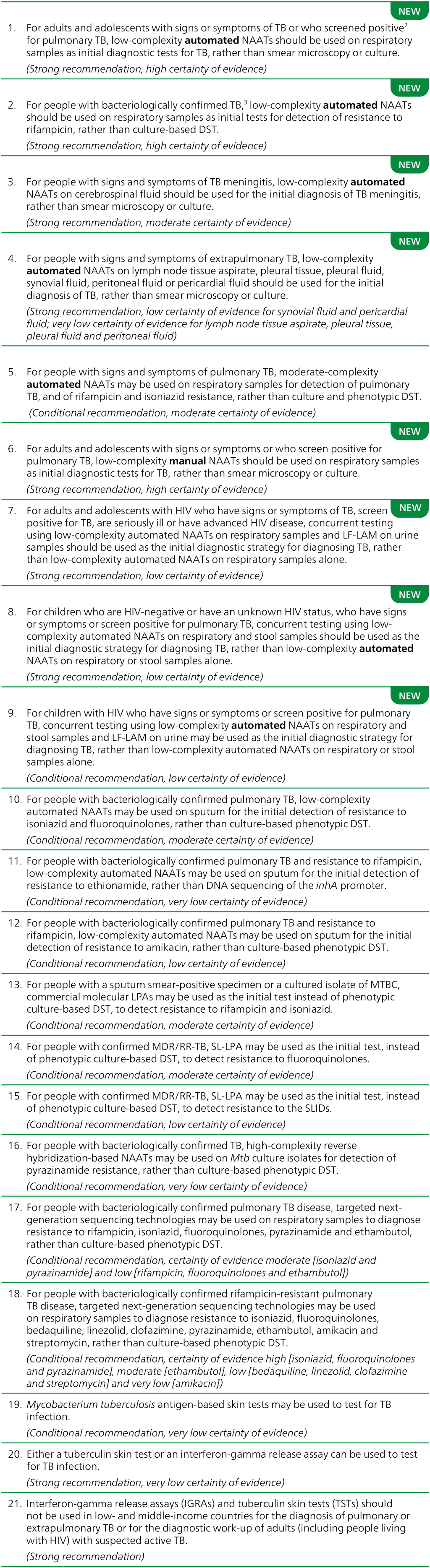
DNA: deoxyribonucleic acid; DST: drug susceptibility testing; HIV: human immunodeficiency virus; LF-LAM: lateral flow urine lipoarabinomannan assay; LPA: line probe assay; MDR/RR-TB: multidrug-resistant or rifampicin-resistant TB; Mtb: Mycobacterium tuberculosis; MTBC: Mycobacterium tuberculosis complex; NAAT: nucleic acid amplification test; NGS: next-generation sequencing; SL-LPA: second-line line probe assay; SLID: second-line injectable drug; TB: tuberculosis; WHO: World Health Organization.
2 Having a positive result of a test, examination or other procedure is used to distinguish people who are highly likely to have TB disease from people who are highly unlikely to have TB. At present, WHO recommends the following as screening tests: chest radiography (chest X-ray; CXR) with or without computer-aided detection (CAD), C-reactive protein in people living with HIV, and molecular WHO-recommended rapid diagnostic test for TB (mWRD).
3 A bacteriologically confirmed TB case is one from whom a biological specimen is positive by smear microscopy, culture or WRD (such as Xpert MTB/RIF Ultra). All such cases should be notified, regardless of whether TB treatment has started (10).

 تعليق
تعليق