كتاب روابط اجتياز لـ منصة منظمة الصحة العالمية لتبادُل المعارف بشأن السل
In many high TB burden settings, sputum smear microscopy remains the primary diagnostic technique for evaluating individuals who present with the signs and symptoms of TB, or screen positive for TB. However, sputum smear microscopy is an insensitive test, with a limit of detection (LoD) of 5000–10 000 bacilli/mL of sputum (16). Furthermore, sputum smear microscopy cannot distinguish drug-susceptible strains from drug-resistant strains. WHO continues to recommend that NTPs replace microscopy as the initial diagnostic test with WRDs that directly detect MTBC.
The current gold standard method for bacteriological confirmation of TB is culture using commercially available liquid media. However, culture is not used as a primary diagnostic test in many high TB burden countries because of the cost, the infrastructure requirements (biosafety level 3 [BSL-3] or TB containment laboratory) and the long time required to generate results (1–3 weeks for a positive result and up to 6 weeks for a negative result). Nevertheless, culture is important for the diagnosis of paediatric and extrapulmonary TB from paucibacillary samples, and in the differential diagnosis of non-tuberculous mycobacteria (NTM) infection. Additionally, both sputum smear microscopy and culture remain necessary to monitor a person’s response to treatment.
The culture process can result in the growth of many different Mycobacterium species. As such, laboratory confirmation of TB requires testing of the recovered mycobacteria using a species identification test (e.g. Capilia™ TB-Neo, Tauns Laboratories, Numazu, Japan; Bioline™ TB Ag MPT64 Rapid Test, Abbott, Gyeonggi-do, Republic of Korea; or TBcID, Becton Dickinson Microbiology Systems, Sparks, United States of America [USA]) to definitively identify MTBC. Species identification is particularly important before initiating phenotypic DST.
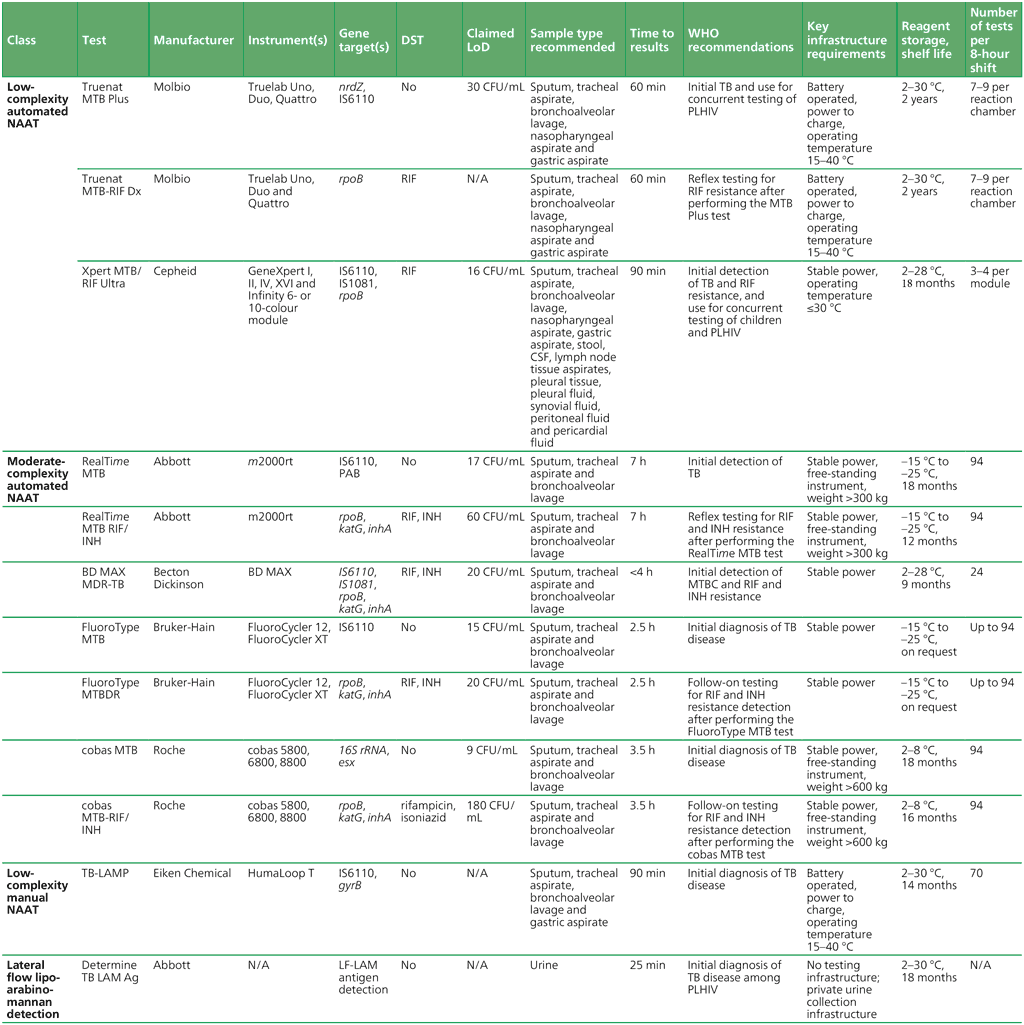
Table 2.2. Summary of initial tests for TB diagnosis

 تعليق
تعليق