كتاب روابط اجتياز لـ منصة منظمة الصحة العالمية لتبادُل المعارف بشأن السل
2.3.1 Low-complexity manual NAATs
The class of LC-mNAATs is defined in Table 2.5. Only one test met the criteria for the class: the Loopamp™ MTBC detection kit from Eiken Chemical Company in Tokyo, Japan. The test detects MTBC but not drug resistance, and it is useful in decentralized settings with limited laboratory infrastructure. New products that are successfully added to the class by WHO PQ can be found in the WHO list of prequalified in vitro diagnostic products (18).
Table 2.5. Definition of the class of LC-mNAATs
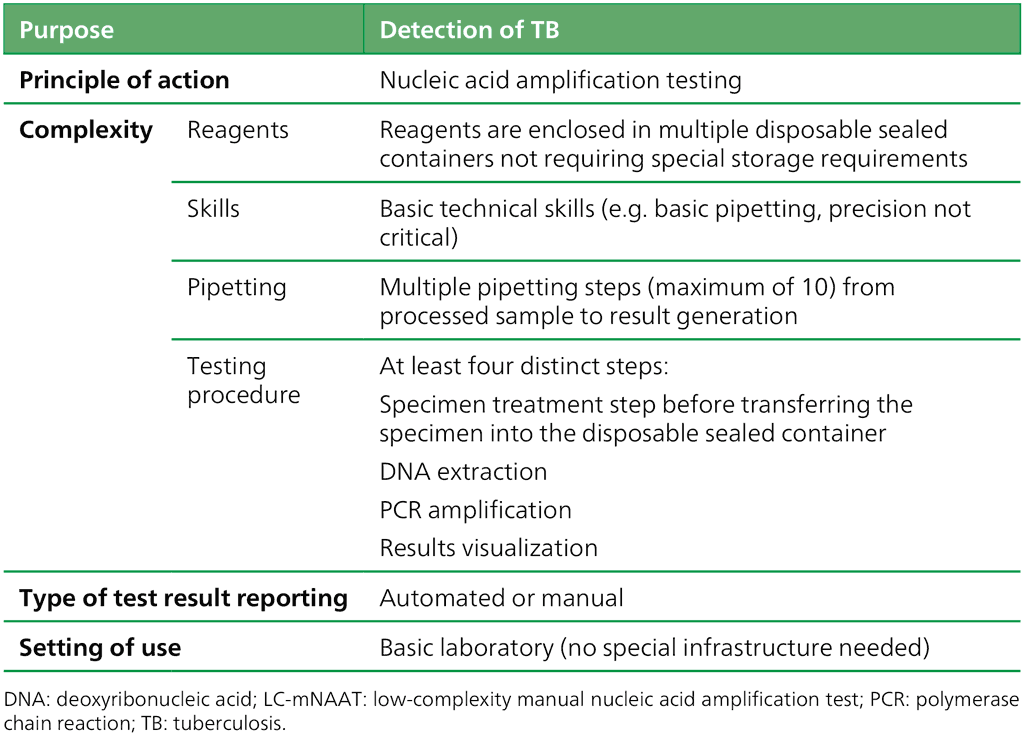
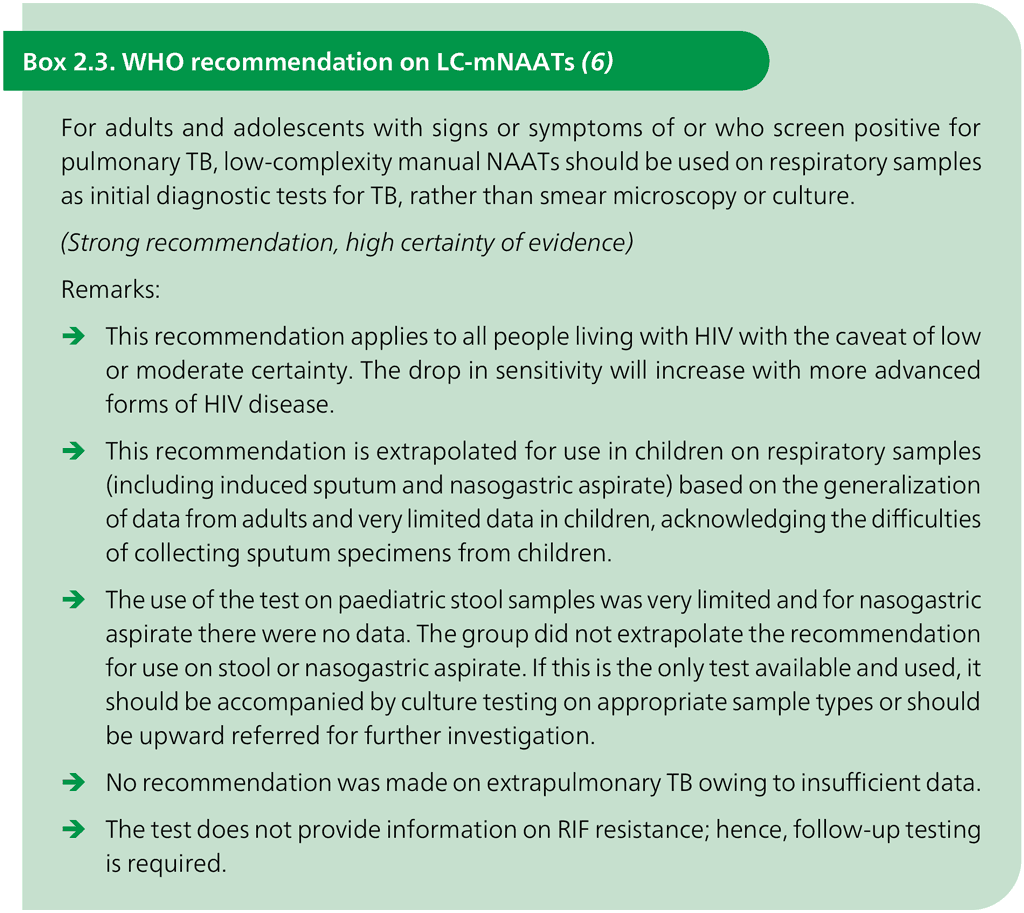
The LC-mNAATs are accurate in detecting pulmonary TB on respiratory samples in adults, children and people living with HIV (Box 2.3). Currently, such tests are only recommended for use with sputum (all ages) and gastric aspirate (children) because evidence on other sample types is lacking (see the section on further research in the consolidated guidelines (5)). The manual TB-LAMP assay is designed to detect MTBC directly from sputum specimens in about 90 minutes, does not require sophisticated instrumentation and can be used at the peripheral laboratory level, with the biosafety requirements being similar to those for sputum smear microscopy. For the detection of TB in adults with signs and symptoms consistent with pulmonary TB, LC-mNAAT demonstrated a sensitivity of 84% (95% credible interval [CrI]: 78–89%) and a specificity of 96% (95% CrI: 94–97%) on sputum, as compared with a microbiological reference standard.
2.3.2 Urine LF-LAM
The urine LF-LAM is an immunocapture lateral flow assay based on the detection of LAM (lipoarabinomannan), which is a component of the mycobacterial cell wall. It is a POC test for the initial diagnosis of TB among people living with HIV. Although the assay lacks sensitivity, it can be used as a fast, rule-in test for HIV-positive individuals that can be used in the community, a health facility or at the bedside, especially in urgent cases where a rapid TB diagnosis is critical for the person’s survival. The Determine TB LAM Ag assay is currently the only commercially available urine LF-LAM endorsed by WHO. The presence of LAM in the urine is an indication of the presence of mycobacteria; hence, WHO has defined a positive LF-LAM result as a bacteriologically confirmed TB case. However, the detection of mycobacterial LAM antigen in urine does not provide any information on drug resistance. A document addressing practical considerations for the implementation of the LF-LAM is available (20).
NEW
The recommendations have been updated to include adults and adolescents living with HIV in all settings (inpatient and outpatient, irrespective of CD4 cell count) and children with HIV (5), as detailed in Box 2.4.

Notes:
- For their initial diagnostic test, anyone with signs and symptoms of pulmonary TB who can produce sputum should have a sputum specimen submitted for concurrent testing with an LC-aNAAT. This includes children and adolescents living with HIV who can provide a sputum sample. LF-LAM results (test time 25 minutes) are likely to be available before mWRD results; hence, treatment decisions should be based on the LF-LAM result while awaiting the results of other diagnostic tests.
- LF-LAM may be used to assist in the diagnosis of TB but it should not be used as a screening test.

 تعليق
تعليق