كتاب روابط اجتياز لـ 1266
This section provides brief descriptions of WHO-recommended technologies for the detection of TB, DR-TB and TB infection. It also summarizes WHO recommendations for such technologies; these recommendations are more thoroughly discussed in the latest versions of the WHO consolidated guidelines on tuberculosis: module 3: diagnosis (5) and the WHO consolidated guidelines on tuberculosis: module 1: prevention (14).
In 2020, WHO changed from product-specific TB testing recommendations to class-based recommendations, with products grouped into classes based on their:
- purpose of use (e.g. detection of Mtb or drug resistance);
- principle of action;
- infrastructure and human resource requirements;
- complexity (i.e. of the testing procedure and associated instrumentation);
- reporting method (automated versus manual); and
- intended setting of use (e.g. reference, peripheral or low-complexity laboratory; POC or near POC).
Since that time, nine classes of TB tests have been established, encompassing more than 25 recommended products.
The full list of classes and their products are organized by their intended use and presented in Table 2.1 as:
- initial tests for diagnosis of TB:
- with drug-resistance detection;
- without drug-resistance detection;
- follow-on drug-resistance tests after bacteriological confirmation of TB; and
- tests for TB infection.
Table 2.1. Classes of TB testing technologies and products included in current guidelines
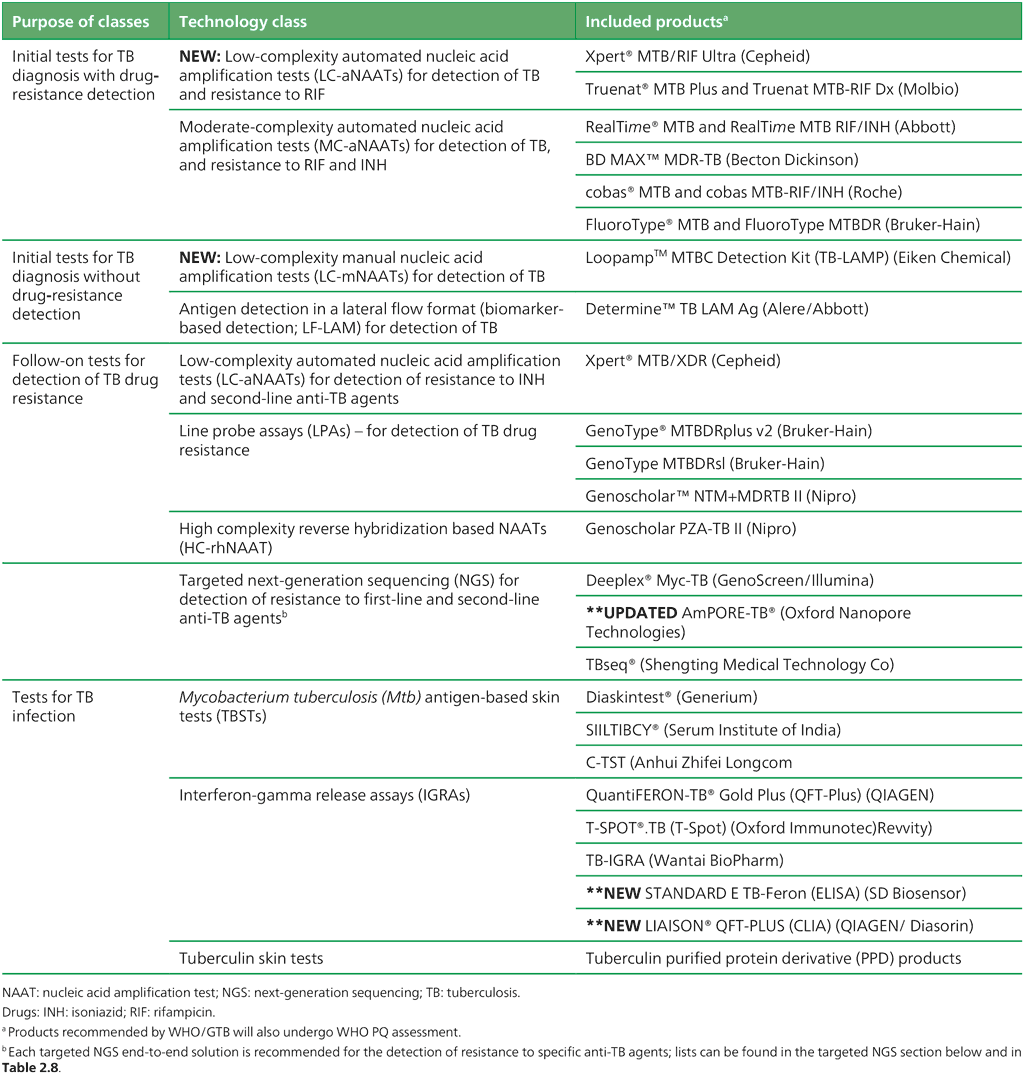
WHO has reviewed each of these tests and solutions and has developed recommendations for their use. In all settings, WHO recommends that rapid diagnostic tests should be used for initial diagnosis of TB and detection of RIF resistance, to minimize delays in starting treatment.
The initial tests for TB diagnosis are broadly grouped as WRDs; these are defined as diagnostic tests that directly target mycobacterial DNA (molecular) or cell components (biomarkers) to aid in the diagnosis of TB (5). The newer, sensitive molecular tests recommended for the initial detection of Mtb complex (MTBC) – with and without initial drug-resistance detection – are mWRDs and are organized into three classes (5):
- the low-complexity automated NAATs (LC-aNAATs) for the detection of MTBC and RIF resistance;
- the moderate-complexity automated NAATs (MC-aNAATs) for the detection of MTBC and RIF and INH resistance; and
- the low-complexity manual NAATs (LC-mNAATs) for the detection of MTBC alone.
Table 2.1 lists the mWRDs that meet the criteria for the respective classes; Table 2.2 provides further details on each test’s manufacturer, instrumentation, infrastructure requirements, targets, times and limits of detection. Further product-specific details may also be found in the GLI mWRD selection manual (15). Of note, policy guidance establishing these LC-mNAAT and LC-aNAAT classes and updating recommendations for use of the included tests was recently updated; hence, Section 3 and Section 4 of this handbook have been updated to reflect the latest changes (5).
In addition to the mWRDs, one biomarker-based LF-LAM WRD – Determine TB LAM Ag – is recommended for diagnosis of TB in people living with HIV. A positive LF-LAM result is considered to be bacteriological confirmation of TB (10). However, a negative result does not rule out TB; therefore, LF-LAM is recommended for concurrent use with mWRD testing among people living with HIV. Policy guidance on the LF-LAM testing was recently updated and is detailed in the WHO consolidated guidelines on tuberculosis: module 3: diagnosis (5), while operational considerations are provided in Section 3 and Section 4 of this handbook. After the initial detection of TB, three classes of follow-on tests are recommended to detect resistance to first-line or second-line anti-TB drugs (or both): LC-aNAATs, line probe assays (LPAs) and targeted NGS end-to-end solutions, see Table 2.1 and Table 2.2. A summary of the aspects of the tests included in the classes is found in Table 2.6; information sheets specific to the targeted NGS solutions are provided in Web Annex A.
To detect when a person is infected with Mtb and may be most likely to benefit from receiving TPT, WHO recommends three classes of TB infection tests: TBSTs, IGRAs and TST. All three classes include tests that target factors associated with a person’s immune response to infection – they do not directly target components of Mtb. Hence, tests for TB infection require a competent immune response to produce valid results, cannot distinguish TB infection from TB disease, and cannot predict who will progress from TB infection to TB disease. Further information on the recommendations for use of these tests can be found in the WHO consolidated guidelines on tuberculosis: module 1: prevention (14). More information concerning operational considerations on testing for TB infection is outlined in Section 2.5 below

 تعليق
تعليق