Перекрёстные ссылки книги для ПЛАТФОРМА ВОЗ ПО ОБМЕНУ ЗНАНИЯМИ О ТБ
3.1 Access to DST
The current guidelines for treatment of DR-TB stress the need for access to reliable, quality-assured DST, to be provided by NTPs and associated laboratories, to inform the use of the WHO-recommended regimens. Rapid molecular testing is making it increasingly feasible for NTPs to quickly detect MDR/RR-TB and other types of resistance, and to use the results to guide treatment decisions (5, 6). Hence, rapid molecular testing should be made available and accessible to ensure DST for at least rifampicin, isoniazid and FQ, given that DST for these drugs is essential for selecting the most appropriate initial DR-TB regimen. If the capacity for rapid molecular testing is lacking, NTPs should promptly build such capacity, and should make efforts to ensure universal access to all patients initiating a regimen for any form of TB, including both drug-susceptible and drug-resistant forms. To meet the End TB Strategy targets, DST for rifampicin and isoniazid should be offered to all TB patients, and all RR-TB patients should be offered DST for FQ. In addition, DST for the drugs used in the newly recommended regimens is increasingly important.
In addition to building capacity to ensure the routine performance of DST for medicines used in the clinical management of all patients, NTPs need to build surveillance systems to determine the local prevalence of DR-TB strains to guide programmatic planning. This is especially important for drugs for which resistance testing is not routinely performed or where the resistance prevalence of the drug is expected to be low initially (e.g. pretomanid) and needs to be monitored over time. DRS can be based on data from routine diagnostic DST in TB patients (i.e. continuous surveillance) or from special surveys representative of the entire TB patient population (i.e. drug-resistance surveys) (7). Data from local TB DRS may provide baseline estimates of the prevalence of resistance, including among relevant subgroups of recurrent and re-registered individuals with TB disease (e.g. recurrence or new episode of TB or return after LTFU). It can also provide monitoring trends to inform DST algorithms and inform broader local policy decisions (1, 8).
WHO recommends using WHO-recommended rapid diagnostics (WRD) as the initial tests to diagnose TB. The WRDs for the initial detection of TB (with or without drug resistance detection) include four classes of tests:
- low-complexity manual nucleic acid amplification tests (LC-mNAATs);
- urinary biomarker-based lateral flow LAM (LF-LAM) tests;
- low-complexity automated NAATs (LC-aNAATs) and
- moderate-complexity automated NAATs (MC-aNAATs).
Currently, only one test – loop-mediated isothermal amplification for detection of Mtb (TB LAMP) – is included in the class of LC-mNAATs, and the sole test – Determine TB LAM Ag – is included in the LF-LAM class, with neither test capable of detecting drug resistance. The class of LC-aNAATs for detection of TB and drug resistance includes the Xpert MTB/RIF Ultra, Truenat MTB Plus and MTB-RIF Dx tests. These tests detect the Mtb complex and resistance to rifampicin. The class of MC-aNAATs includes multiple tests produced by a range of manufacturers (Abbott, BD, Bruker/Hain Lifesciences, and Roche) that are used as initial tests to detect Mtb complex and resistance to rifampicin and isoniazid. This class of tests is suitable for intermediate to central laboratories due to their infrastructural requirements. However, the initiation of a pertinent TB treatment regimen should not be delayed while waiting for DST results; hence, it is critical to ensure access to WRDs for the initial detection of TB at all levels of the health care system, either through onsite testing or through a functional sample transportation system.
A person with confirmed rifampicin-susceptible or rifampicin-resistant TB should be tested for isoniazid resistance to ensure appropriate treatment. The MC-aNAATs provide this result upfront but follow-on molecular tests recommended by WHO (i.e. Xpert MTB/XDR, line probe assay [LPA] and targeted next-generation sequencing [NGS]) can detect resistance to isoniazid, FQ, pyrazinamide, ethionamide and injectable drugs. Except for the first-in-class of the follow-on LC-aNAATs – the Xpert MTB/XDR that detects resistance to isoniazid, FQ, ethionamide and injectable drugs – the follow-on tests are more complex and require specialized infrastructure, qualified staff, biosafety conditions and a well-functioning sample transportation system. The first-line LPA detects resistance to rifampicin and isoniazid, the second-line LPA detects resistance to FQ and injectable drugs, and the first-in-class test of high-complexity hybridization NAATs detects resistance to pyrazinamide. The limited availability of LC-aNAATs and MC-aNAATs makes routine isoniazid resistance testing challenging in many settings.
The follow-on class of targeted NGS can detect resistance to rifampicin, isoniazid, pyrazinamide, ethambutol, FQ, bedaquiline, linezolid, clofazimine, amikacin and streptomycin. Tese technologies are best suited for use at the reference laboratory level. Targeted NGS can provide DST results in a few days, excluding sample transportation and reporting (9).
The interpretation of the results of molecular DST may be complex and it includes multiple steps. For resistance to isoniazid, mutations in two genes (inhA and katG) are interrogated. If a mutation is present only in inhA, it is likely that isoniazid can still be effective at a high dose, whereas a mutation in katG alone or mutations in both inhA and katG render isoniazid no longer effective, even at a high dose (10). For many anti-TB drugs, only selected mutations are known or can be detected by molecular tests; thus, depending on the type of the test, risk of resistance in a particular patient and result, the use of culture-based DST may still be needed. This would apply in the case of a negative LPA result for isoniazid, FQ or injectable drug resistance, especially if prevalence of resistance to these drugs is high or resistance is suspected in a particular patient. Similarly, in the case of negative result for resistance to bedaquiline, linezolid and clofazimine by targeted NGS and a high risk of resistance to these drugs in a particular setting or patient, the use of culture-based DST may still be needed. Further details on diagnostic tests recommended can be found in the relevant WHO consolidated guidelines (9) and operational handbook (11) in Module 3: Diagnosis – rapid diagnostics for tuberculosis detection.
Country programmes need to work towards the establishment of phenotypic DST for all TB medicines for which there are now agreed reliable and reproducible methods (e.g. bedaquiline, clofazimine, delamanid, FQ, isoniazid, linezolid and rifampicin). The critical concentrations for various drugs were either established for the first time (bedaquiline, clofazimine, delamanid, linezolid, pretomanid and cycloserine) (12) or revised (rifampicin and FQ) in WHO technical consultations (13). Resistance to ethionamide/prothionamide may be inferred from the results of molecular testing for isoniazid resistance (i.e. presence of mutations in the inhA promotor region) using either automated or manual molecular tests. However, susceptibility to ethionamide/prothionamide cannot be inferred purely on the basis of the absence of a mutation in the inhA promotor gene using commercially available NAATs, because resistance can be conferred by other mutations in the inhA gene and its promotor and by mutations in the ethA gene that are not detected by these NAATs. A standardized phenotypic DST method using the mycobacterial growth indicator tube (MGIT) liquid culture automated system has been recently developed for pretomanid and cycloserine. Critical concentrations for pretomanid and cycloserine are included in the third edition of the operational handbook in Module 3: Diagnosis – rapid diagnostics for tuberculosis detection. Phenotypic DST for ethambutol, ethionamide/prothionamide, imipenem/meropenem or p-aminosalicylic acid is not routinely recommended because results may be unreliable (10).
The inability to undertake DST routinely in all patients despite all possible efforts should not be a barrier to starting patients on a potentially life-saving MDR-TB regimen; however, treatment should always be considered in a context of the potential risk of prescribing ineffective treatment and amplifying drug resistance, with a subsequent decrease in the likelihood of treatment effectiveness. If DST for bedaquiline and linezolid is not yet available, the clinician or the TB programme manager needs to estimate the likelihood of effectiveness of the medicines used, informed by the patient’s history of use of TB medicines, the drug-resistance pattern of the contact or source case, and recent representative DRS data. A reliable clinical history of exposure to bedaquiline and linezolid should thus be considered when designing a treatment regimen; however, this should be the main source of evidence to guide regimen design only in situations where phenotypic DST is not yet available. For paediatric patients, it is not always possible to obtain a DST result, owing to the difficulty of obtaining an adequate specimen or the lack of bacteriological confirmation; hence, the treatment regimen design should be based on the drug-resistance pattern of the index case. In the absence of individual DST, relevant population surveillance data are essential to inform the choice and design of MDR-TB treatment regimens. In addition to TB DRS findings, it is important for practitioners to know which medicines have been in frequent use in each geographical setting or patient groups. If DST is not routinely available for individual patients where there is treatment failure, storage of Mtb isolates collected at baseline or during treatment monitoring can be considered for performing phenotypic DST or whole genome sequencing at reference laboratories.
The adoption and implementation of a new regimen can and should proceed while the DST capacity is being established.
Despite some of the uncertainties about DST, NTPs should strive to test for resistance to a wide set of TB drugs and offer the most appropriate treatment regimen. The patient’s clinical response to treatment should always be carefully monitored. If there is a poor treatment response, undiagnosed drug resistance or heteroresistance (i.e. the coexistence of susceptible and resistant organisms in the same patient) should be considered, as should alternative explanations for failure to respond to treatment, such as poor or erratic adherence to treatment, malabsorption, inadequate patient education or support, IRIS or the presence of comorbidities (14).
3.2 Safety monitoring and management and provision of patient support
All treatment offered to people with MDR/RR-TB should align with WHO-recommended standards, including patient-centred care and support, informed consent where necessary, principles of good clinical practice, active TB drug-safety monitoring and management (aDSM), and regular patient monitoring to assess regimen effectiveness. Health care providers must offer careful clinical and bacteriological follow-up to assess the TB treatment response, with general laboratory support to monitor and manage AEs and comorbidities. The provision of social support is essential to enable adherence to treatment (15). Certain programmatic components (e.g. aDSM) (15, 16) are recommended for all patients on any MDR/RR-TB regimen (Annex 3). An appropriate schedule of laboratory tests and clinical examinations should be included in the patient’s treatment chart to identify AEs (15). In settings where aDSM has not yet been fully rolled out and national guidelines have not been updated, patients should not be left to wait until all programme components are fully in place before they can receive potentially life-saving interventions. The WHO consolidated guidelines also reinforce the message that patient support is critical for good treatment adherence and improved outcomes (17).
3.3 Extensive pulmonary TB disease
For patients with extensive pulmonary disease, non-medical interventions (e.g., resection surgery) and respiratory support measures should be prioritized alongside medical treatment. Additionally, these patients should be thoroughly evaluated for post-tuberculosis lung disease (PTLD) following the completion of MDR/RR-TB treatment. PTLD is a globally under-recognized condition associated with reduced life expectancy and an increased risk of recurrent tuberculosis, underscoring the need for comprehensive post-treatment care and monitoring. (18).
3.4 Regimen options in the treatment of DR-TB
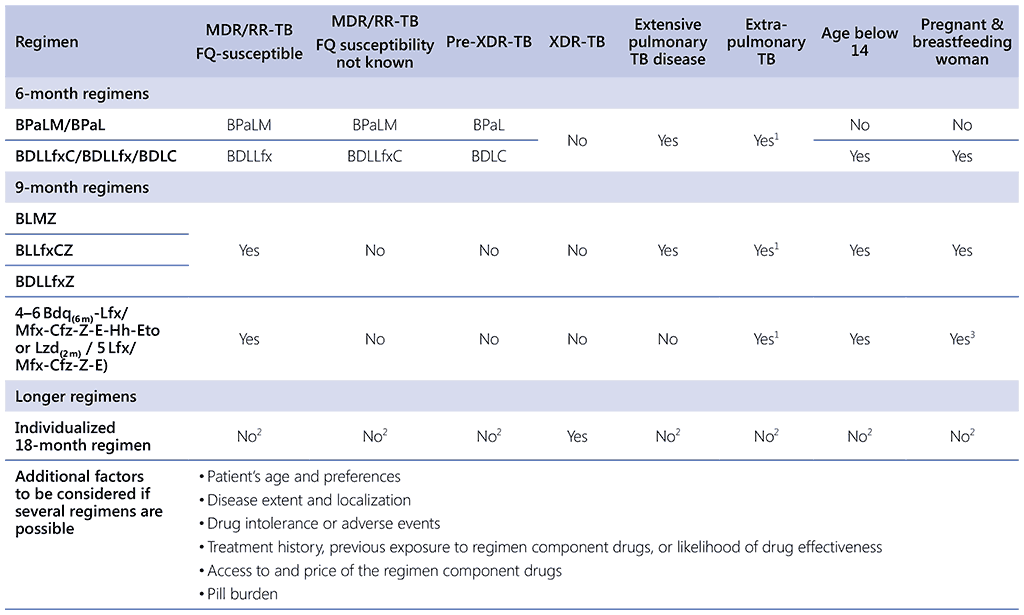
In patients with MDR/RR-TB, several regimens are available based on current WHO guidelines. Key factors that influence the choice of treatment regimen include the drug-resistance profile, previous exposure to TB medicines, the patient’s health history, the drug-resistance profile of close contacts, the patient’s age and preferences, pregnancy status and the extent and localization of TB disease: pulmonary or extrapulmonary, with central nervous system (CNS) involvement or disseminated. The overall preference is for shorter, safer, better tolerable and more effective treatment regimens.
The evidence suggests that the balance of effects probably favours shorter and simpler regimens when compared with the standard of care (SoC); that is, 9-month or longer regimens. However, in cases of confirmed or presumed severe forms of extrapulmonary DR-TB (e.g. those with CNS involvement or dissemination to multiple organ systems) the treatment approach may require clinical judgement by an experienced specialist. These cases often necessitate longer regimens, inpatient care and, where there is CNS involvement, the inclusion of additional medicines with demonstrated efficacy in penetrating the blood–brain barrier. Among other considerations, the choice of regimen should be carefully tailored to the patient’s clinical condition and the extent of disease dissemination, ensuring optimal treatment outcomes.
A shorter duration of treatment is preferred by patients and presents several advantages. For example, it decreases the risk of LTFU; is less of a burden on the patient, their household and the health system; and minimizes health care costs and unemployment stress.
Among available options, 6-month regimens are preferred over the 9-month or 18–20-month regimens, and 9-month regimens are preferred over the 18–20-month regimens.
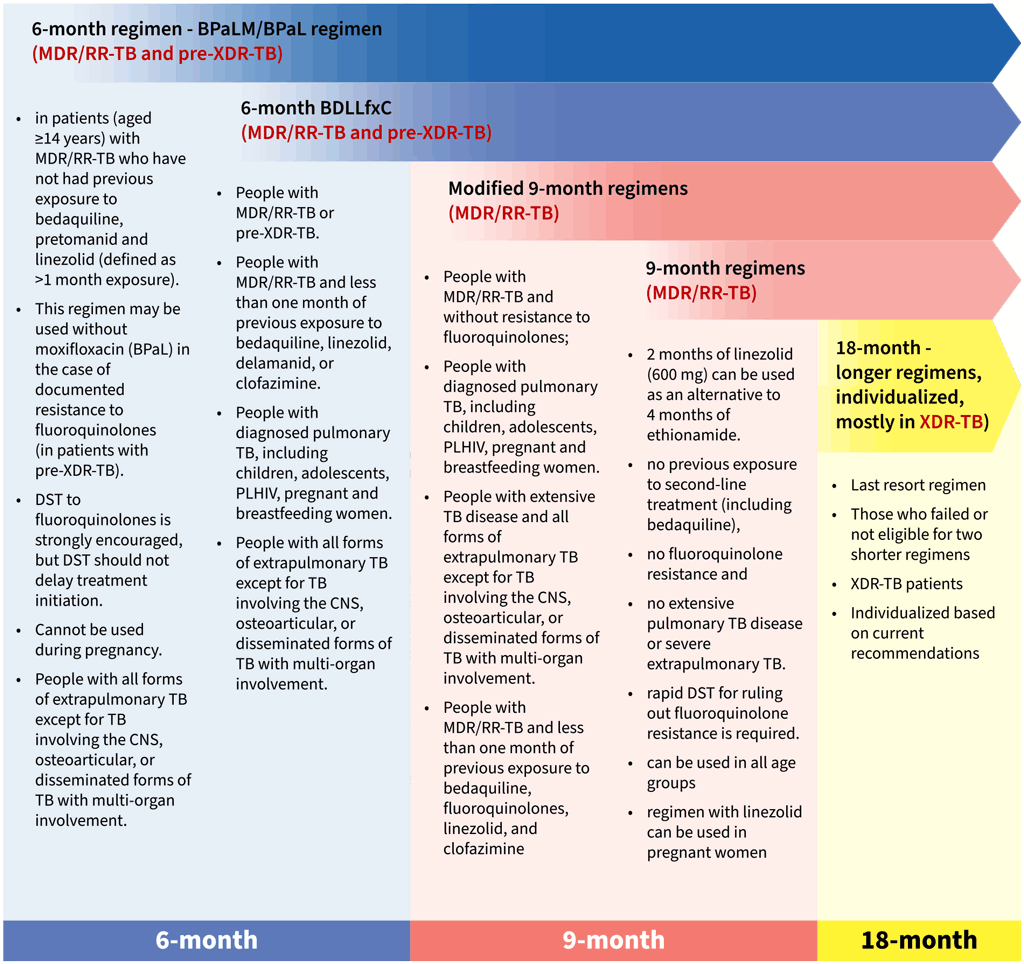
- The 6-month BPaLM regimen (6 Bdq-Pa-Lzd-Mfx12) – comprising bedaquiline, pretomanid, linezolid and moxifloxacin – is a preferred regimen for adults and adolescents aged 14 years and older and is recommended for patients with MDR/RR-TB or pre-extensively drug-resistant TB (pre-XDR-TB). Patients with unknown FQ resistance can be initiated on the BPaLM regimen while waiting for DST results. In cases where resistance to FQ (pre-XDR-TB) is identified before or after initiation of treatment, moxifloxacin can be omitted and the BPaL regimen can be initiated or continued, because there is probably no added benefit of using a drug with demonstrated resistance that may have toxicities. The duration of the BPaLM regimen is largely standardized for 6 months (26 weeks), whereas BPaL can be extended to a total of 9 months (39 weeks). It is suitable for people with confirmed pulmonary TB and all forms of extrapulmonary TB, except for cases involving the CNS, osteoarticular TB and disseminated (miliary) TB. The regimen is applicable regardless of HIV status. It is not recommended during pregnancy.
- The 6-month BDLLfxC regimen (6 Bdq-Dlm-Lzd-Lfx-Cfz) – comprising bedaquiline, delamanid, linezolid, levofloxacin and clofazimine – is an alternative regimen for individuals with MDR/RR-TB or pre-XDR-TB and may be used for children, adolescents below 14 years of age and pregnant or breastfeeding women. Depending on the availability of FQ DST results, the regimen may be used with or without levofloxacin or clofazimine. In cases of unknown FQ resistance, BDLLfxC can be initiated without delay; BDLLfx is used for FQ-susceptible TB, and BDLC (bedaquiline, delamanid, linezolid and clofazimine) for FQ-resistant TB. Treatment is typically for 6 months (24 weeks) but may be extended by an additional 3 months, for a total duration of up to 9 months (36 weeks).¹³ This regimen may be offered to individuals with any extent of pulmonary TB disease, as well as most forms of extrapulmonary TB, except for cases involving the CNS, osteoarticular TB or disseminated forms with multiorgan involvement.
Table 2.3.1. Regimen options and factors to be considered for selection of treatment regimens for patients with MDR/RR-TB
1 except for CNS TB, osteoarticular TB & disseminated TB with multi-organ involvement.
2 should not be used unless shorter regimen options are not available.
3 Only Lzd variation: 4–6 Bdq(6 m)-Lfx/Mfx-Cfz-Z-E-Hh- Lzd(2 m) / 5 Lfx/Mfx-Cfz-Z-E).
In patients with MDR/RR-TB and in whom resistance to FQ has been excluded, modified 9-month regimens (BLMZ, BLLfxCZ and BDLLfxZ) are available and are generally preferred over longer (18- month) regimens. These regimens comprise bedaquiline in various combinations with levofloxacin/moxifloxacin, linezolid, clofazimine, delamanid and pyrazinamide. These regimens may be used for children, adolescents below 14 years of age and pregnant or breastfeeding women. Available options include the following:
- BLMZ is the first choice among the recommended modified 9-month regimens. In terms of the balance of health effects it is preferable to both BLLfxCZ and BDLLfxZ. Also, at the time of the review, it had a lower pill burden, fewer AEs, balanced efficacy and cost and appeared either preferable or equivalent for all other decision criteria. It comprises bedaquiline, linezolid, moxifloxacin and pyrazinamide.
- BLLfxCZ is the second option if BLMZ is unsuitable. BLLfxCZ, compared to BDLLfxZ has a similar but slightly preferable balance of health effects, significantly lower cost and a lower pill burden than BDLLfxZ. It comprises bedaquiline, linezolid, levofloxacin, clofazimine and pyrazinamide. Although effective, it is considered second because of the inclusion of clofazimine, which affects the safety of this regimen.
- BDLLfxZ is the third option for patients who cannot use BLMZ or BLLfxCZ. It comprises bedaquiline, delamanid, linezolid, levofloxacin and pyrazinamide. Although delamanid has a favourable safety profile, its current high price and limited Phase 3 trial data make this regimen less favourable.
If none of the above regimens can be used, an alternative option is the previously recommended 9-month regimen, which offers variations incorporating ethionamide or linezolid.
- 9-month regimens (4–6 Bdq(6 m)-Lfx/Mfx-Cfz-Z-E-Hh-Eto or Lzd(2 m) / 5 Lfx/Mfx-Cfz-Z-E): These regimens can be used in patients with MDR/RR-TB and in whom resistance to FQ has been excluded. Therefore, access to rapid DST is required before starting a patient on one of these regimens. The 9-month regimens are not appropriate for patients with extensive TB disease, and only linezolid containing variation can be used for pregnant and breastfeeding women. The 9-month all-oral regimen comprises bedaquiline (used for 6 months) in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high dose), pyrazinamide and clofazimine (for 4 months, with the possibility of extending to 6 months if the patient remains sputum smear positive at the end of 4 months), followed by treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (for 5 months). Ethionamide can be replaced by 2 months of linezolid.
If none of the previously mentioned treatments are viable, the final, last resort option would be to use the individualised, longer regimens.
- Longer, individualized regimens (≥18 months): These regimens are reserved for patients with MDR/RR-TB who do not eligible for, or have not responded well to, the 6- or 9-month regimens; they are also used for patients with XDR-TB or drug intolerance. These regimens are individualized based on drug-resistance profiles, hierarchical grouping of second-line TB medicines, treatment history and patient characteristics. The regimens typically last at least 18 months and are used as a last resort.
There is no evidence directly comparing the two 6-month regimens, so the choice cannot be guided by evidence alone. However, various eligibility factors and significant price differences between these regimens can help NTPs in making their decision on which regimen to adopt and include in their national guidelines. For children, adolescents under 14 years of age, and pregnant or breastfeeding women, the BDLLfxC is the only 6-month regimen option available. This regimen should be prioritized for these groups. In cases where MDR/RR-TB patients have FQ resistance excluded and are not eligible for 6-month regimens, 9-month regimens should be considered.
Decisions on an appropriate regimen should be made based on likely efficacy, safety, patient preference and clinical judgement, also taking into account the results of DST, patient treatment history, age, severity and site of the disease (Table 2.3.1 and Fig. 2.3.1).
Fig. 2.3.1. Regimen options for MDR/RR-TB treatment
MDR/RR-TB: multidrug-resistant or rifampicin-resistant tuberculosis.
12 The regimen notations used throughout this document highlight the number of months for which a relevant combination of medicines is used; where certain drugs are used for a different duration, this is also noted, using subscript in brackets.
13 The duration of treatment (24 weeks or 36 weeks) is aligned with the evidence available from the clinical trial. However, if the national programmes find it challenging to implement, they may choose to standardize the duration across regimens, e.g., 26 weeks for 6 months and 39 weeks for 9 months.

 Обратная связь
Обратная связь