Перекрёстные ссылки книги для 1266
This section refers to the treatment regimens for MDR/RR-TB that have standardized durations of 9 months with oral agents. Section 5.1 describes the seven-drug regimen, which utilizes linezolid or ethionamide, referred to as the “9-month regimen”. Section 5.2 introduces a new set of three four- or five-drug regimens recently recommended by the WHO (1), collectively referred to as the “modified 9-month regimens”. All the 9-month regimens are administered orally.
Table 2.5.1. Overview of 9-month regimens
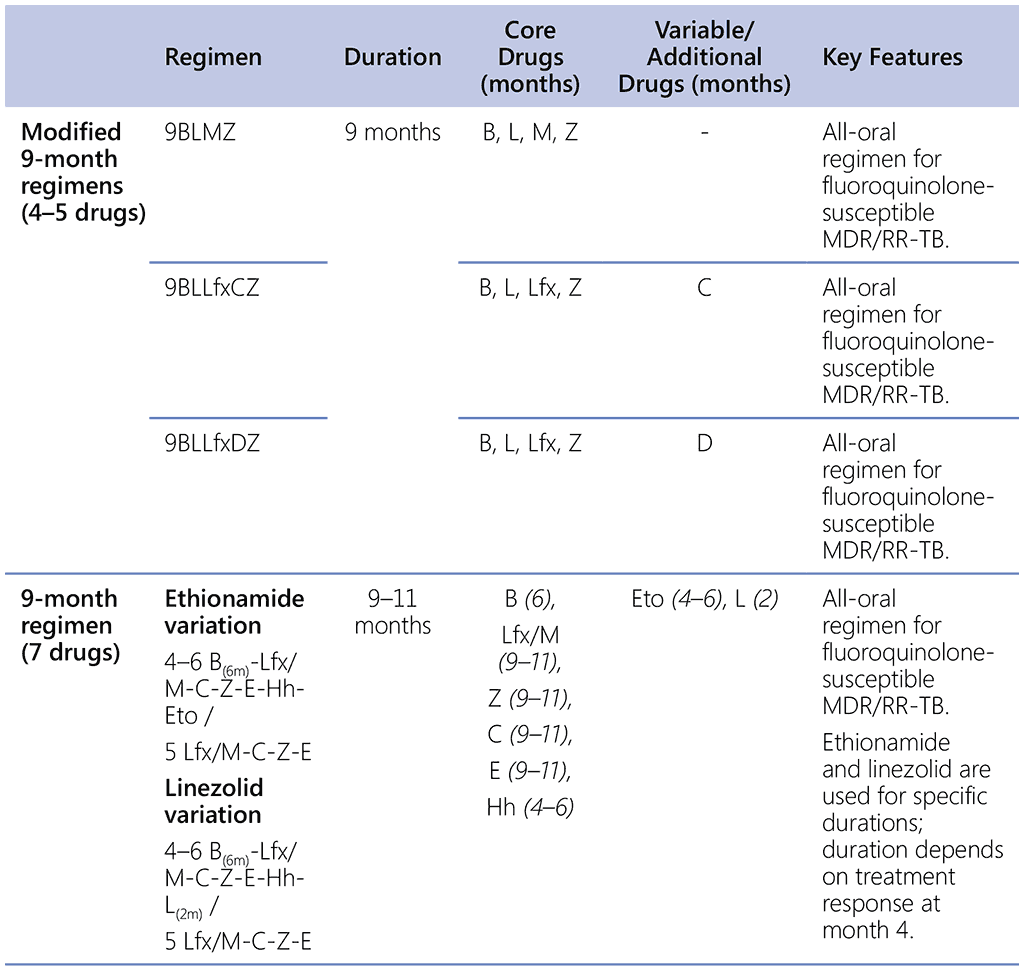
B: bedaquiline; C: clofazimine; D: delamanid; E: ethambutol; Eto: ethionamide; Hh: high-dose isoniazid; L: linezolid; Lfx: levofloxacin; M: moxifloxacin; Z: pyrazinamide.
5.1 The 9-month all-oral regimen for MDR/RR-TB
This section refers to a treatment regimen for MDR/RR-TB that has a duration of at least 9 months and uses a seven-drug, bedaquiline-containing combination that includes either linezolid or ethionamide. The GDG reviewed the evidence for this regimen during the 2019, 2020 and 2022 guideline updates. The recommendation in the consolidated 2025 guidelines (1) states:

Remarks
- The 9-month all-oral regimen consists of bedaquiline (used for 6 months), in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high-dose), pyrazinamide and clofazimine (for 4 months, with the possibility of extending to 6 months if the patient remains sputum smear positive at the end of 4 months), followed by treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (for 5 months). Ethionamide can be replaced by 2 months of linezolid (600 mg daily).
- A 9-month regimen with linezolid instead of ethionamide may be used in pregnant women, unlike the regimen with ethionamide.
- This recommendation applies to:
- people with MDR/RR-TB and without resistance to fluoroquinolones;
- patients without extensive TB disease and without severe extrapulmonary TB;
- patients with less than 1 month exposure to bedaquiline, fluoroquinolones, ethionamide, linezolid and clofazimine; when exposure is greater than 1 month, these patients may still receive this regimen if resistance to the specific medicines with such exposure has been ruled out;
- all people regardless of HIV status;
- children (and patients in other age groups) who do not have bacteriological confirmation of TB or resistance patterns but who do have a high likelihood of MDR/RR-TB (based on clinical signs and symptoms of TB, in combination with a history of contact with a patient with confirmed MDR/RR-TB).
In 2019, for the WHO guideline update, the South African Department of Health provided WHO with access to programmatic data on injectable-free regimens that had been used in South Africa since 2017, when most eligible patients were enrolled on a shorter regimen, with bedaquiline replacing the injectable. Since then, new data from patients receiving WHO-recommended shorter and longer MDR/RR-TB regimens have been incorporated into an individual patient dataset (IPD) in 2021; these data were presented to the GDG in 2022 to help inform the development of the updated WHO guidelines on DR-TB. In addition, routine data from the South African NTP were available to assess the 9-month all-oral regimen containing linezolid (600 mg daily) instead of ethionamide, for treatment of patients with FQ-susceptible MDR/RR-TB and without previous exposure to second-line TB drugs. The South African routine dataset of patients receiving the 9-month all-oral linezolid-containing regimen would also have excluded children aged below 6 years, as well as patients with extensive pulmonary TB disease and severe forms of extrapulmonary TB, because these patients were not considered eligible for this regimen under national DR-TB guidelines (52). For the updated 2022 WHO guidelines, the 9-month linezolid-containing regimen used in South Africa was compared with the earlier dataset and the 2021 IPD from patients meeting the same eligibility criteria and who received the WHO-recommended 9-month all-oral regimen containing ethionamide instead of linezolid, or the longer regimens designed based on the 2020 WHO recommendations.
Following review of the data presented, the GDG judged the benefits of the 9-month regimen with linezolid to be small and the undesirable effects to be moderate compared with the 9-month regimen with ethionamide. The certainty of evidence was judged to be very low. Based on this, the GDG judged that the balance of health effects does not favour either the 9-month regimen with linezolid or the 9-month regimen with ethionamide. Therefore, WHO has updated its conditional recommendation that, in eligible patients with MDR/RR-TB, the 9-month all-oral regimen may be used, and that 2 months of linezolid can be used as an alternative to 4 months of ethionamide within this shorter regimen (1).
The implementation of these two variations of the 9-month all-oral regimen (i.e. including either linezolid for 2 months or ethionamide for 4 months) is to provide more flexible and effective treatment options for MDR/RR-TB. This regimen still requires combined use of seven agents (some with considerable toxicity), most of which will be continued for at least 9 months. Patients will need support to overcome the hardships associated with TB and its treatment, including daily adherence challenges, adverse drug reactions, indirect costs and stigma.
5.1.1 Eligibility
In settings where the 6-month MDR/RR-TB regimen is not yet available, or implementation of the regimen is not yet feasible, or for patients who are not eligible, selected patients with MDR/RR-TB may benefit from a 9-month all-oral regimen. Several eligibility criteria must be considered for this regimen, with additional considerations for the use of linezolid instead of ethionamide.
The 9-month all-oral regimen (with either ethionamide or linezolid) may be offered to the following patients with MDR/RR-TB (where resistance to at least rifampicin has been confirmed and resistance to FQ has been ruled out):
- those with no documented resistance or suspected ineffectiveness of bedaquiline, clofazimine, or ethionamide or linezolid (whichever is considered for inclusion in the regimen);
- those with no exposure to previous treatment with bedaquiline, FQ,¹⁹ clofazimine, or ethionamide or linezolid (whichever is considered for inclusion in the regimen) for more than 1 month – when prior drug exposure is greater than 1 month, patients may still receive this regimen if resistance to the specific medicine with such exposure has been ruled out;
- those with no extensive or severe TB disease²⁰ and no severe extrapulmonary TB;²¹
- all people living with or without HIV;
- women who are pregnant or breastfeeding: and
- children and adults without bacteriological confirmation of TB or resistance patterns but who require MDR/RR-TB treatment based on clinical signs and symptoms of TB (including radiological findings) and history of contact with someone with confirmed MDR/RR-TB: these patients may be eligible for this regimen based on the drug resistance profile of the isolate obtained from the most likely index case.
Linezolid is associated with considerable toxicity, which necessitates close monitoring for signs of bone marrow suppression and neuropathies. Optic neuritis and peripheral neuropathies tend to be reported beyond 2 months of treatment with linezolid, whereas myelosuppression is significantly dose dependent and is more likely to occur during the first 2 months of exposure to the drug (53, 54). Nevertheless, linezolid is far more effective than ethionamide and helps to maintain a relatively effective regimen, particularly in cases of MDR/RR-TB where phenotypic DST results are awaited to confirm FQ susceptibility. Therefore, the 9-month all-oral regimen containing linezolid (instead of ethionamide) should be offered wherever possible to patients who fulfil the eligibility criteria above, as well as the following:
- serum Hb above 8 g/dL, neutrophils above 750 mm³ and platelets above 150 000/mm³ at the start of treatment; and
- no evidence of severe peripheral neuropathy, or any sign or suspicion of optic neuritis, at the start of treatment.
The 9-month all-oral regimen with ethionamide instead of linezolid, or a longer regimen without linezolid, may be more appropriate options for patients with very low Hb, neutrophils or platelets, severe peripheral neuropathy or concerns regarding vision. Mild or moderate peripheral neuropathy (Grade 1 or 2) may also be sufficient reason to offer a 9-month regimen that uses ethionamide, based on the patient’s preference after discussing the risks and benefits of not including linezolid. However, the ethionamide-containing regimen must be avoided during pregnancy. The decision regarding which regimen offers the best option for cure in a patient may also depend on other considerations; for example, preferences of patients and clinicians, pill burden, drug formulations, regional DRS data, feasibility of monitoring for drug adverse effects, and availability of blood transfusion services or ophthalmology services, if required.
5.1.2 Composition, dosing and duration of the regimen
The two variations of the 9-month all-oral MDR/RR-TB regimen recommended by WHO (1) are described below.
Ethionamide variation
The ethionamide variation involves the initiation of bedaquiline, levofloxacin/moxifloxacin, clofazimine, ethionamide, ethambutol, isoniazid (high dose) and pyrazinamide. All seven drugs are given for 4 months, with the possibility of extending to 6 months if the patient’s sputum remains bacteriologically positive at the end of the fourth month on treatment. Ethionamide and high-dose isoniazid are dropped after 4 or 6 months, depending on the decision to extend treatment based on smear status at month 4 of treatment. This is followed by 5 months of treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide. Bedaquiline is usually given for 6 months but could be extended to 9 months, particularly if the initial phase is extended from 4 to 6 months due to a lack of sputum conversion at month 4.
The regimen is summarized as:
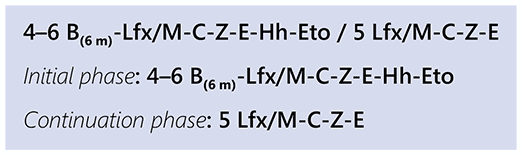
B: bedaquiline; C: clofazimine; D: delamanid; E: ethambutol; Eto: ethionamide; Hh: high-dose isoniazid; L: linezolid; Lfx: levofloxacin; M: moxifloxacin; Z: pyrazinamide.
Linezolid variation
The linezolid variation involves initiation of bedaquiline, linezolid, levofloxacin/moxifloxacin, clofazimine, ethambutol, isoniazid (high dose) and pyrazinamide. Linezolid is only given for the first 2 months of treatment. Clinical and haematological monitoring are crucial to detect early linezolid-associated AEs, particularly haematological events (sudden or significant drop in Hb, neutrophils or platelets). After the initial 2 months, the remaining six drugs are given for another 2 months (with the possibility of extending by an additional 2 months if the patient’s sputum remains bacteriologically positive at the end of the fourth month on treatment). High-dose isoniazid is dropped after 4 or 6 months, depending on the decision to extend treatment based on smear status at month 4 of treatment. This is followed by 5 months of treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide. Bedaquiline is usually given for 6 months but could be extended to 9 months, particularly if the initial phase is extended from 4 to 6 months due to a positive sputum smear result at month 4.
The regimen is summarized as:

B: bedaquiline; C: clofazimine; D: delamanid; E: ethambutol; Eto: ethionamide; Hh: high-dose isoniazid; L: linezolid; Lfx: levofloxacin; M: moxifloxacin; Z: pyrazinamide.
Choice of fluoroquinolone
In terms of choice of FQ, either levofloxacin or moxifloxacin may be used in the 9-month all-oral regimen, because they have shown similar efficacy for treating MDR/RR-TB. Although levofloxacin results in a higher pill burden, it is often preferred because moxifloxacin is associated with a higher risk of QT interval prolongation (55). Clinically significant, severe QT interval prolongation is relatively uncommon among patients treated with the 9-month all-oral regimens. However, the additive effect of co-administration of other QT-prolonging drugs (i.e. bedaquiline and clofazimine) within the shorter regimen should be considered when deciding on an appropriate regimen for individual patients with other risk factors for cardiotoxicity.
Dosing and frequency
The dosages of all drugs included in both variations of the 9-month all-oral regimen are outlined in the Annex 4. Most drugs, except for bedaquiline, are administered once a day, 7 days per week. In the 9-month regimen, bedaquiline is initially administered daily, with a higher loading dose for the first 2 weeks, followed by a lower maintenance dose on 3 days a week (with at least 48 hours between doses) thereafter. Alternatively, daily dosing of bedaquiline can also be considered. If one dose of bedaquiline is missed in the 2-week loading phase, the missed dose does not have to be made up and the patient can continue on the daily dosing schedule. If a dose of bedaquiline is missed in the maintenance phase but is remembered within that 48-hour dosing period, the dose should be administered as soon as possible, and the following dose adjusted to be taken 48 hours later, with resumption of the usual thrice-weekly dosing schedule thereafter. For example, if bedaquiline is dosed every Monday, Wednesday and Friday, then if the Wednesday dose is missed it can still be taken on Thursday, and then the following dose should be taken on Saturday, with a return to the usual dosing schedule on Monday. If bedaquiline is interrupted for more than 2 weeks (but <8 weeks) during the maintenance phase of dosing, the drug should be reloaded at the higher daily dose for 7 days before resuming the thrice-weekly dosing schedule. If bedaquiline is interrupted for less than 2 consecutive weeks during the maintenance phase, no reloading is required. If bedaquiline is interrupted for more than 8 consecutive weeks, then the patient and treatment plan should be reassessed because the patient will no longer be eligible to continue or restart the 9-month all-oral regimen.
5.1.3 Modifications of treatment
The 9-month all-oral MDR/RR-TB regimen should be implemented as a standardized package. It is not advisable to change the composition of the regimen or the duration of either the initial or continuation phase, with a few exceptions, as follows:
- Bedaquiline is usually given for 6 months but may be extended to 9 months if the initial phase of the regimen is extended from 4 to 6 months because of positive sputum smears at month 4 of treatment.
- Linezolid is only given for 2 months (instead of 4–6 months of ethionamide). If occasional doses of linezolid are missed during that time, the missed doses can be added on to the end of the 2-month period if the patient is tolerating the drug well; however, once FQ resistance has been definitively ruled out, it may not be strictly necessary to make up the missed doses. The linezolid dose should not be reduced to less than the recommended dose to reduce the severity of adverse effects. If the full dose of linezolid (600 mg in adults) is not tolerated for the first full 2 months of treatment (apart from occasionally missed doses, which can be added to the end of the 2-month period), then the patient must either switch to an ethionamide-containing 9-month regimen (provided FQ susceptibility is confirmed and the patient is not pregnant) or to an individualized longer regimen without linezolid. In selected cases where the risk of undetected resistance to FQ and other second-line TB drugs is very low and the patient is unable to tolerate linezolid but would greatly benefit from a shorter regimen (e.g. migrant populations and children), the treating clinician may, after weighing up the risks and benefits, choose to stop linezolid before 2 months and continue the 9-month all-oral regimen, with close monitoring for relapse or recurrence.
- Prothionamide may be used instead of ethionamide.
- Moxifloxacin may be used instead of levofloxacin, provided close ECG monitoring is feasible (should this be required).
- If, for any reason, a patient is unable to tolerate pyrazinamide or ethambutol within the 9-month regimen, then one (but only one) of these drugs may be dropped during the continuation phase without necessitating a switch to a longer regimen. If two or more of these drugs are not tolerated within the 9-month regimen, the treatment will have to switch to a longer regimen. If any of the other drugs within the 9-month regimen (bedaquiline, levofloxacin/moxifloxacin, linezolid/ethionamide or clofazimine) are stopped early because of toxicity or intolerance then the patient will also have to switch to a new regimen. Patients switching to a new regimen due to toxicity or intolerance need to be reported as “treatment failed” (Section 10, Chapter 1).
- At the fourth month of treatment on the 9-month regimen, the decision to extend the initial phase from 4 to 6 months is based on the bacteriological sputum smear status of the patient’s sputum specimen. If the specimen is smear negative at month 4 (regardless of smear status at the start of treatment), the patient may move to the continuation phase of treatment. If the specimen is smear positive at month 4, the initial phase is prolonged to 6 months. The duration of the continuation phase remains fixed at 5 months.
- At the sixth month of treatment, the culture result from the specimen taken at month 4 and possibly month 5 should be available, as well as the smear results from the specimens taken at months 5 and 6. If the culture from the 4-month specimen is positive for Mtb, the clinician should undertake a full work-up to assess for the risk of treatment failure – this involves a comprehensive clinical assessment, review of treatment adherence to address specific challenges, radiological assessment and collection of another respiratory sample for bacteriological assessment, as well as repeat DST of the most recent positive culture to test for emerging resistance to second-line TB drugs. Similarly, if the month 5 and 6 culture results remain persistently positive, treatment failure should be suspected, particularly if the patient has had suboptimal adherence to treatment or shows other signs of poor clinical or radiological response to treatment.
Discontinuation and change to another treatment regimen
If a patient starts the 9-month all-oral MDR/RR-TB regimen but is later found to be ineligible following detection of Mtb resistance to FQ, the patient must change to a different regimen. Such patients might be eligible for a 6-month BPaL or BDLC regimen if their prior exposure to bedaquiline and linezolid was for less than 1 month and there is no demonstrated resistance to any components of the BPaL or BDLC regimen. The BPaL regimen may only be considered if the patient meets the eligibility criteria and the regimen is available and feasible in the setting. In cases where an eligible patient starts the 9-month all-oral MDR/RR-TB regimen but additional resistance is detected later in treatment (after initial DST indicated susceptibility to Group A and B drugs), it can be assumed that further acquisition of resistance may have emerged during that period of drug exposure; such patients should be considered for a treatment outcome of failure and should not continue with the 9-month regimen. The 6-month BPaL or BDLC regimen should not be offered to these patients because amplification may have occurred to linezolid and bedaquiline, key drugs in both the BPaL and BDLC regimens. The patient should switch to a longer individualized regimen, with repeated phenotypic DST to guide the composition of the longer regimen.
Patients who start a 6-month BPaLM or BDLLfxC regimen may change to the 9-month all-oral regimen, if required, provided they meet the necessary eligibility criteria for the 9-month regimen. This may be warranted when toxicity to linezolid develops early in the BPaLM or BDLLfx regimen and necessitates a linezolid-sparing regimen, such as the 9-month regimen with ethionamide.
Patients who start on a longer regimen but are subsequently found to be eligible for the 9-month all-oral regimen may change to the 9-month regimen if this is done within the first month of starting treatment. There is little experience in changing from longer to shorter regimens in this way; hence, clinical monitoring and adequate data collection are important to inform future treatment recommendations.
5.1.4 Key subgroups
People living with HIV
The 9-month all-oral MDR/RR-TB regimen was evaluated in a setting with a high HIV prevalence. In the dataset analysed for the 2022 WHO guidelines, over 70% of patients starting a shorter regimen were also living with HIV, and among those, more than 90% were receiving ART. A 9-month all-oral regimen is expected to perform similarly in PLHIV who initiate ART early, in accordance with WHO recommendations. However, clinicians should be mindful of the overlapping, additive toxicities and potential DDIs with ARV medicines and TB drugs. Co-administration of zidovudine and linezolid should be avoided because of the increased risk of myelosuppression. Boosted protease inhibitors can increase bedaquiline exposure, thereby increasing the risk of bedaquiline-related adverse drug reactions (e.g. QT interval prolongation), which may require closer monitoring. Efavirenz can reduce the concentration of bedaquiline; therefore, this antiretroviral drug should be avoided in patients receiving the 9-month all-oral regimen. There are no overlapping toxicities or DDIs with dolutegravir in patients receiving the shorter regimen with either linezolid or ethionamide. PLHIV receiving the 9-month all-oral regimen will need prophylactic medication for opportunistic infections, support for adherence to TB and antiretroviral medication, and close monitoring of the biomarkers of immune status.
Children
Although only six patients aged below 14 years were included in the analysis of the shorter regimen dataset from South Africa, the evidence supporting the use of this regimen in adults may be extrapolated to younger patients, provided the implementation considerations are followed. The benefits of a shorter regimen for a child with MDR/RR-TB should be weighed against the high pill burden and the difficulties of administering each of the seven drugs in this regimen, particularly if child-friendly formulations of the drugs are not available. Bedaquiline is relatively well tolerated and easy to administer to children; adult-formulation bedaquiline tablets suspended in water have been shown to have the same bioavailability as tablets swallowed whole (Annex 4).
Aside from bedaquiline, the medicines that compose the 9-month all-oral regimen have been part of MDR/RR-TB regimens for many years, in similar combinations, for both adults and children. The associated adverse drug reactions have been widely described and the drug dosages established (Annex 4). Child-friendly formulations are now available for all second-line drugs and should be provided to children whenever possible. When these are not available, practical instructions for use of adult formulations for administration are available, so lack of formulation should not be a hindrance to treating children of all ages. This must be addressed as a priority by treatment programmes that include management of children with MDR/RR-TB.
With dosing and safety data available for use of bedaquiline in children aged below 6 years, the removal of the age restriction for the use of bedaquiline means that children of all ages with MDR/RR-TB may be offered the 9-month all-oral regimen if they meet the eligibility criteria (56). Extent of disease is defined slightly differently for children than for adults, and most children with TB have less severe forms of the disease than adults.
Evidence from the SHINE trial (Shorter Treatment for Minimal Tuberculosis in Children), which was the first and only large Phase 3 trial to evaluate the duration of TB treatment in children with non-severe drug-susceptible TB (DS-TB), suggests that pulmonary TB disease should be classified as severe (which may include extensive, advanced and complicated disease) or non-severe in children (56). Despite the lack of comparable data among children with MDR/RR-TB disease specifically, the same definitions for severity of disease are likely to be appropriate when considering the use of a shorter regimen for children with MDR/RR-TB. Non-severe disease in children is defined as peripheral lymph node TB; intrathoracic lymph node TB without airway obstruction; uncomplicated TB pleural effusion (without empyema or pneumothorax); or paucibacillary, non-cavitary disease confined to one lobe of the lungs and without a miliary pattern (evaluated on chest X-ray) (56).
Bacteriological confirmation of MDR/RR-TB disease in younger children is relatively uncommon, and the decision to treat for MDR/RR-TB may rely on clinical signs and symptoms, radiological findings and significant exposure to someone with microbiologically confirmed MDR/RR-TB. In children without microbiological confirmation of TB disease or rifampicin resistance, the choice of regimen relies partly on the drug-resistance pattern of the isolate obtained from the most likely index case.
For most second-line TB drugs, adverse effects appear to be less frequent in children than in adults; however, close monitoring is still warranted in children, regardless of the regimen. Before a shorter regimen containing linezolid is offered to a child, the clinician must consider the feasibility of close monitoring, particularly for haematological side-effects, which requires repeated blood draws for at least the first 2 months of treatment. Visual acuity and colour vision are more difficult to monitor in younger children than in older children and adults. Ethionamide might be considered a safer alternative for children in some settings, but this should be balanced against the lower efficacy of the drug compared with linezolid, and the poor gastrointestinal tolerability and need to monitor for hypothyroidism.
Pregnant and breastfeeding women
Dosing and safety data to support the optimal use of second-line TB medicines during pregnancy are generally sparse. There have been case reports and observational data reporting successful treatment and pregnancy outcomes among women who received treatment (including bedaquiline-containing regimens) for MDR/RR-TB during pregnancy and postpartum, but pregnant and breastfeeding women are usually excluded from clinical drug trials and early access programmes. Even less is known about the effects of MDR/RR-TB treatment on the infant in-utero and after birth; however, in general, the benefits (to both parent and child) of providing effective MDR/RR-TB treatment to the parent far outweigh the potential risks posed to the fetus in-utero or the breastfed infant.
Ethionamide is usually contraindicated in pregnancy because animal reproduction studies have shown an adverse effect on the fetus, and there are no adequate and well-controlled studies in humans. The physiologic effects of pregnancy, which lead to a relatively low Hb (due to the dilutional effect of increased blood volume) and a higher risk of peripheral neuropathies, may be exacerbated by the adverse effects of linezolid. Nevertheless, the 9-month all-oral regimen including linezolid instead of ethionamide may be considered for pregnant and breastfeeding patients who meet the eligibility criteria for the shorter regimen with linezolid, although closer monitoring is required.
More compelling evidence on the dosing and safety of specific anti-TB drugs among pregnant and breastfeeding women is needed to guide decision-making on the most appropriate regimen for treatment of MDR/RR-TB during pregnancy and postpartum. In addition, this population group requires considerable adherence support and monitoring of proper administration of MDR/RR-TB treatment, along with other chronic medications, to ensure successful treatment outcomes and minimal risk of TB transmission from mother to infant postpartum. Care providers must also pay particular attention to seamless continuity of care between antenatal and TB services, which are rarely integrated in most TB-endemic settings.
Extensive TB disease
Extensive (or advanced) TB disease in adults is defined as the presence of bilateral cavitary disease or extensive parenchymal damage on chest radiography. In patients aged below 15 years, extensive (or advanced) disease is usually defined by the presence of cavities or bilateral disease on chest radiography. This highlights the importance of chest radiography as part of the diagnostic work-up for patients, along with the usual patient–clinician interaction. Patients with extensive MDR/RR-TB disease should not be treated with the 9-month all-oral regimen with either linezolid or ethionamide because of the lack of evidence on the impact of this regimen in this subgroup of patients.
Older patients
TB-related morbidity and mortality tends to be higher among older people than in the younger population. Patients aged 65 years and older with MDR/RR-TB are more vulnerable to the adverse effects of TB medications owing to physiological changes of ageing (e.g. increase in QT interval and decrease in estimated GFR [eGFR]), other comorbidities and overlapping, additive drug toxicities (owing to a higher likelihood of polypharmacy in older people). Advanced age has also been reported as a risk factor for linezolid-induced anaemia (57). Whereas the 9-month all-oral regimen may be offered to eligible patients of any age, older people may require closer monitoring for drug-related AEs as well as closer adherence support and assistance to administer treatment daily or as prescribed.
Extrapulmonary TB
The dataset evaluated for the 2022 WHO guidelines included patients with uncomplicated extrapulmonary MDR/RR-TB disease. No evidence was available to discern the impact of the 9-month all-oral regimen with either linezolid or ethionamide in patients with severe extrapulmonary TB (defined in this document as the presence of miliary TB or TB meningitis). Although this definition does not specifically include osteoarticular or pericardial TB, a longer treatment regimen may be more suitable in these cases of extrapulmonary TB because of the relatively poor perfusion of TB drugs into the pericardial space and the lack of data on the efficacy of shorter MDR/RR-TB regimens in these cases. The 9-month all-oral regimen should not be offered to patients with severe or complicated extrapulmonary MDR/RR-TB disease. In children, extrapulmonary forms of disease other than lymphadenopathy (peripheral nodes or isolated mediastinal mass without airway compression) are considered severe (56) and may not be adequately treated with the 9-month all-oral regimen.
Patients with co-morbidities (other than HIV)
Patients with diabetes mellitus
The 9-month all-oral regimen may be used to treat MDR/RR-TB in patients with diabetes; however, there are currently no data on safety and outcomes of this regimen in this specific group. Type 2 diabetes is associated with several liver disorders; therefore, it is prudent to monitor closely for hepatotoxicity among these patients. Blood sugar levels may be difficult to control in patients with MDR/RR-TB and diabetes, and insulin may be required to gain adequate blood sugar control during treatment. Patients with diabetes are also at increased risk of peripheral neuropathies, which may be further exacerbated following exposure to linezolid and high doses of isoniazid. These patients must be counselled to report symptoms of peripheral neuropathies early because such symptoms may necessitate a change in regimen – either to the ethionamide-containing 9-month regimen (bearing in mind this will still include high doses of isoniazid in the initial phase), or a longer individualized regimen without linezolid.
Patients with hepatic dysfunction
The 9-month all-oral regimen may not be the most appropriate option for people with CLD because this regimen contains several potentially hepatotoxic drugs (e.g. pyrazinamide, isoniazid and ethionamide). Although this regimen may still be offered with close monitoring of liver enzymes in people with chronic stable liver dysfunction, a longer regimen with fewer hepatotoxic drugs may be preferable in some settings where closer monitoring is not feasible.
Patients with renal failure
The 9-month all-oral regimen may be used to treat MDR/RR-TB in patients with renal failure provided the dose or dosing interval of renally excreted drugs are adjusted for the patient’s creatinine clearance. Levofloxacin (but not moxifloxacin), ethambutol and pyrazinamide require dose or frequency adjustment for adults with creatinine clearance of less than 30 mL/min. Treatment does not have to be extended unless indicated by lack of smear conversion at month 4 of treatment, as for patients with normal renal function.
Patients with anaemia
Patients with TB commonly have anaemia of chronic disease (58), and treatment with an effective drug regimen (even one that includes linezolid) may lead to improvement or resolution of the anaemia once the disease is properly treated. Many patients with TB also suffer with nutritional deficiencies, and low Hb may also be a result of iron deficiency and low iron stores (59). This deficiency may resolve naturally once effective TB treatment (even including linezolid) leads to resolution of TB symptoms and improvement in the patient’s diet and appetite. Extended use (≥2 weeks) of linezolid has been associated with reversible myelosuppression (60). Therefore, the linezolid-containing 9-month regimen must not be offered to patients with a pretreatment serum Hb below 8 g/dL that cannot be rapidly corrected (i.e. with blood transfusions) before starting MDR/RR-TB treatment. Similarly, owing to the morbidity associated with severe neutropenia and thrombocytopenia, the linezolid-containing 9-month regimen is not suitable in patients with neutrophils below 750/mm³ or platelets below 150 000/mm³ before starting treatment. Some patients respond well to an initial blood transfusion that raises their Hb above 8 g/dL and allows them to at least start a linezolid-containing regimen – linezolid will not necessarily cause myelosuppression in patients with baseline anaemia, although a baseline Hb below 10.5 g/dL has been reported as a risk factor for linezolid-induced anaemia (57). It is not uncommon for Hb to drop again shortly after blood transfusion in a person with untreated chronic TB disease, but the temporary increase in Hb may allow enough time for a linezolid-containing regimen to be effective in treating the TB disease, and the patient’s Hb is likely to improve as the disease is brought under control.
Blood transfusions may not be a lasting solution in situations where Hb drops significantly from baseline because of linezolid toxicity when linezolid is continued. Although blood transfusions may help to reverse anaemia following withdrawal of linezolid, they may not resolve linezolid-induced myelosuppression with ongoing exposure to the drug. Therefore, if linezolid toxicity leads to a drop in Hb below 8 g/dL during the first 2 months of treatment, linezolid should be withdrawn and the regimen switched appropriately. More research is needed on the role of iron supplementation to treat anaemia during MDR/RR-TB treatment; however, oral supplementation of iron is often not well tolerated and is not immediately effective at the start of treatment, at a time when the pill burden can be overwhelming and the risk of multiple drug side-effects is high.
5.1.5 Implementation considerations
DST results
The 9-month all-oral regimen is not adequate for the treatment of patients with pre-XDR-TB or XDR-TB; it is also not adequate to treat MDR/RR-TB that has both inhA and katG mutations. Therefore, DST is recommended at or before the start of this regimen to exclude resistance to at least FQ and to determine the mutations conferring resistance to isoniazid.
There are rapid DST methods available for pyrazinamide (LPA, targeted NGS), bedaquiline, clofazimine and linezolid (targeted NGS), which should be performed where available before start of the 9-month regimen. In addition, the critical concentrations for MGIT have been established, enabling NTPs to perform phenotypic DST. If resistance to these drugs is detected , the 9-month regimen should not be offered, or the patient must switch to a longer individualized treatment regimen. In the absence of DST for bedaquiline, linezolid and clofazimine, treatment decisions will rely on the likelihood of effectiveness of these medicines, based on an individual patient’s clinical history and surveillance data from the country or region. This should be considered a last resort and an interim measure until the capacity for DST for these drugs becomes available.
Ideally, molecular (genotypic) DST (targeted NGS) for clofazimine, linezolid and bedaquiline should be performed at the time of treatment initiation. However sensitivity of this method for above-mentioned three drugs is suboptimal, that is why if negative results is obtained, but resistance in a particular patient is suspected, phenotypic, culture-based DST still should be performed. Further details on treatment modifications and follow-on DST for MDR/RR-TB patients based on results from targeted NGS can be found in operational handbook in Module 3: Diagnosis – rapid diagnostics for tuberculosis detection (11).
The low-complexity automated nucleic acid amplification test (Xpert MTB/XDR) detects mutations associated with resistance to isoniazid, FQ, second-line injectable drugs and ethionamide in a single test and can be used in the decentralized settings. The targeted NGS solutions can detect resistance in rifampicin, isoniazid, pyrazinamide, ethambutol, FQ, bedaquiline, linezolid, clofazimine, amikacin and streptomycin, but only can be used in centralized, reference setting.
The first and second-line LPAs, are widely used to detect mutations conferring resistance to isoniazid, rifampicin, FQ, amikacin, as well as pyrazinamide; however, in future this test may be replaced by the Xpert MTB-XDR assay and/or targeted NGS in some settings.
In settings without access to the Xpert MTB-XDR cartridge, an LPA (MTBDRplus) can be used to detect the two most common mutations that confer resistance to isoniazid. These mutations are found in the inhA promoter and katG regions, and they confer resistance to isoniazid at different levels. Low-level isoniazid resistance is conferred when only inhA mutations are present, and high-level resistance is conferred when mutations in the katG gene are present. Mutations at the inhA promoter region are also associated with resistance to ethionamide and prothionamide. High doses of isoniazid (15–20 mg/kg) are generally considered to be effective in the presence of low-level isoniazid resistance when used as part of combination therapy, but the efficacy of high doses of isoniazid in the presence of katG mutations remains unclear. Nevertheless, high-dose isoniazid is always included in the 9-month all-oral regimen if either (but not both) of the mutations is present. The presence of mutations in both regions (i.e. inhA promoter and katG genes) suggests that neither isoniazid at a high dose nor thioamides may be effective and therefore the 9-month all-oral regimen is not appropriate in these cases. In the South African setting, the detection of both mutations in MDR-TB strains was considered a surrogate marker for more extensive drug resistance at the time that the 9-month linezolid-containing regimen was introduced. Patients in South Africa who had MDR-TB with both mutations were not considered eligible for the 9-month regimen, and so were not included in the routine dataset presented to the GDG for review. Thus, the efficacy of the 9-month regimen in such cases is largely unknown. In the absence of information on isoniazid resistance or mutation patterns in the case of an individual patient, knowledge of the prevalence of both mutations among locally circulating RR-TB strains (e.g. from DRS in the relevant epidemiological setting) may also inform decisions as to which treatment regimen would be most appropriate. DST for ethambutol is not carried out routinely in most settings. Results of DST for ethambutol and pyrazinamide do not affect eligibility for the 9-month all-oral regimen.
The 9-month regimen is standardized and all seven drugs (including either ethionamide or linezolid) should be initiated from the outset. Pyrazinamide and ethambutol are inexpensive and generally well tolerated, and they may still be efficacious against MDR/RR-TB in people who are eligible for this regimen. However, some health care providers and patients, particularly children and their caregivers, might find the high pill burden posed by these medicines challenging despite the shorter duration of this treatment regimen.
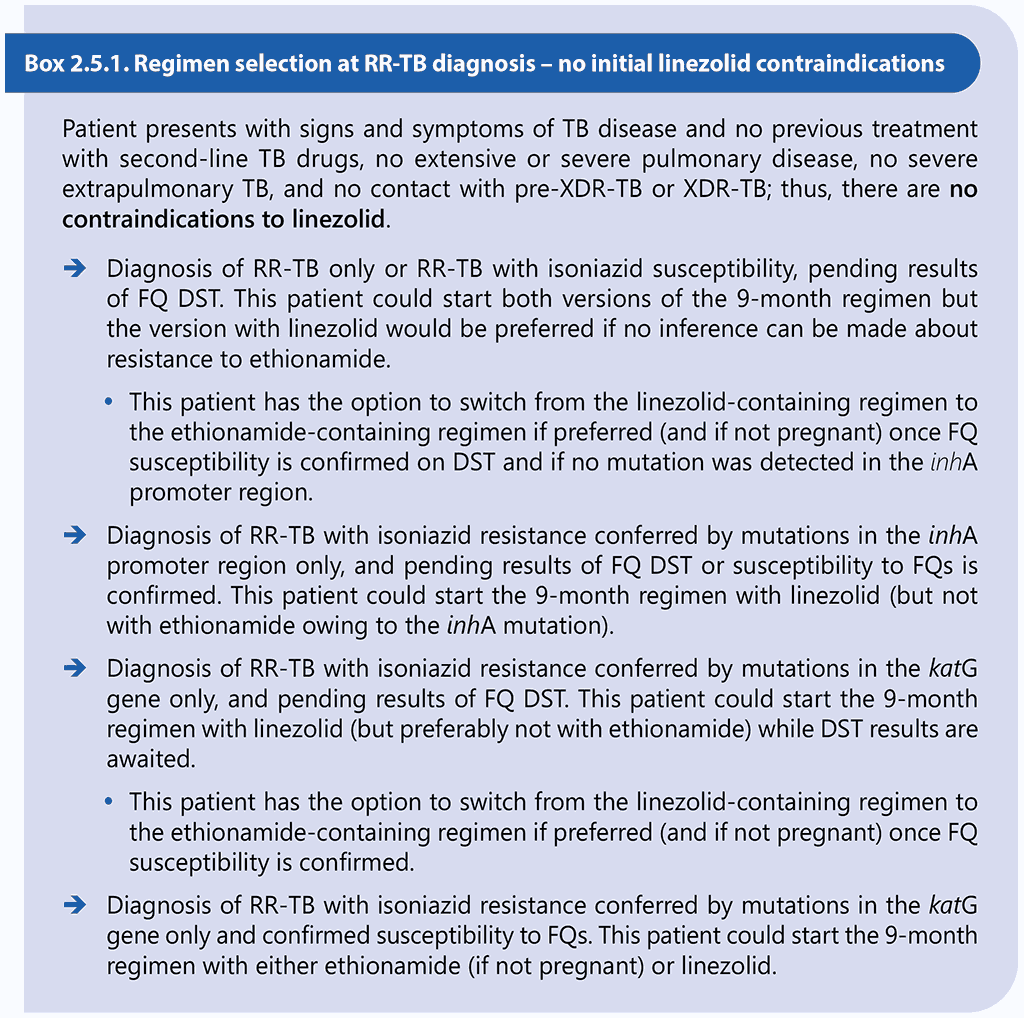
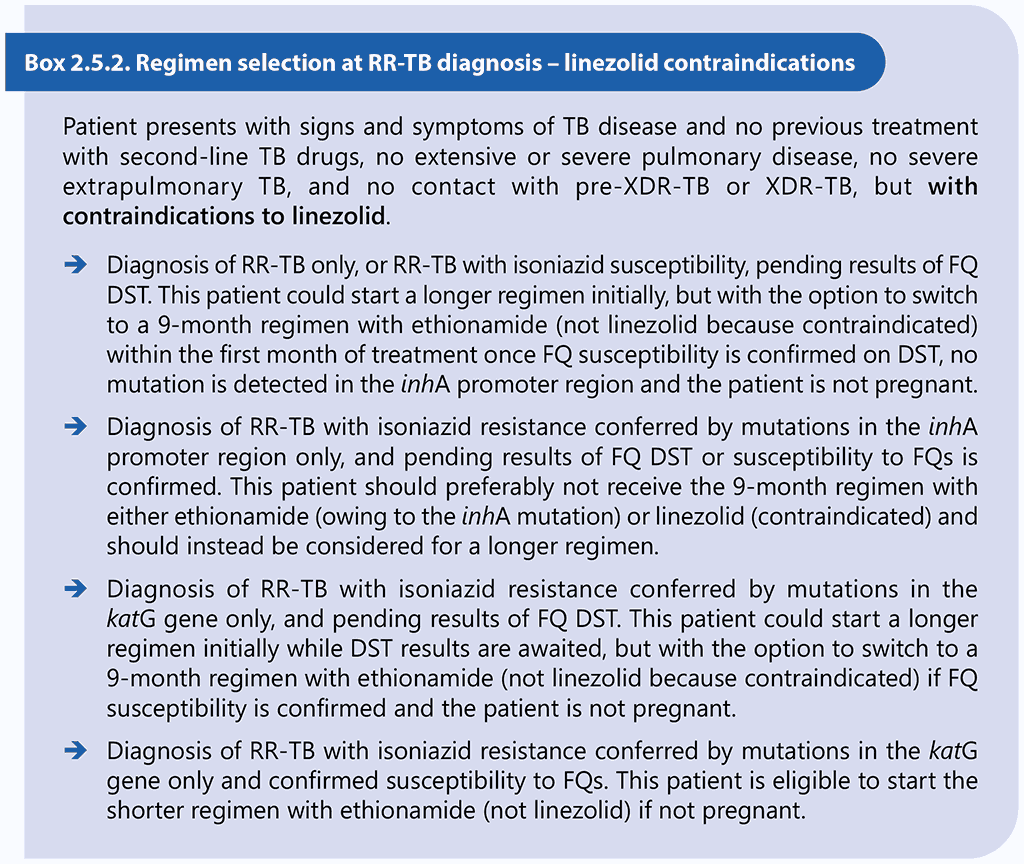
Results of DST should not delay the start of an appropriate MDR/RR-TB treatment regimen, particularly if DST relies on phenotypic methods (61, 62). Box 2.5.1 and Box 2.5.2 below provide examples of clinical scenarios and appropriate choices of regimens.
TB programmes must rapidly build the capacity to undertake DST, and all efforts must be made to ensure access to approved tests. If routine genotypic and phenotypic DST for clofazimine, linezolid and bedaquiline is not feasible for all patients diagnosed with MDR/RR-TB, then DST for these drugs must be prioritized for patients with positive TB sputum cultures at month 4 of treatment or beyond. Delayed conversion to negative cultures, or reversion to positive cultures, after 4 months of MDR/RR-TB treatment may be an early indication that treatment is failing, and clinicians must consider the possibility of acquired drug resistance (63).
Assessment of extent and severity of TB disease
The extent of a patient’s TB disease is important in determining appropriate regimen options, in addition to the drug susceptibility of the Mtb and other considerations mentioned above. Patients with extensive disease are not eligible for the 9-month all-oral regimen with either linezolid or ethionamide.
Patients with severe extrapulmonary MDR/RR-TB are not eligible for a 9-month regimen with either linezolid or ethionamide. Poor penetration of first-line TB drugs through the pericardial tissues leads to low pericardial drug concentrations compared with plasma (64), and although definitive data are lacking, drug penetration remains a concern for second-line TB drugs also. Treatment outcomes among patients treated with longer regimens for osteoarticular MDR/RR-TB are encouraging (65), and there is evidence that linezolid penetrates bone tissue well (66). However, due to the general lack of data on the efficacy of shorter regimens for treatment of these extrapulmonary manifestations of MDR/RR-TB, it remains prudent to treat such patients with longer regimens. In children and young adolescents aged below 15 years, extrapulmonary forms of disease other than lymphadenopathy (peripheral nodes or isolated mediastinal mass without airway compression) are considered to be severe (56) and might not be adequately treated with the 9-month all-oral ethionamide- or linezolid-containing regimen.
Haematological assessment
Due to the risk of myelosuppression associated with even relatively short exposures to linezolid, pretreatment assessment of Hb, neutrophils and platelets is crucial in patients considering treatment with a linezolid-containing regimen. Severe anaemia in patients with TB is a significant risk factor for poor treatment outcomes (67), and patients with a low baseline Hb may be at risk of severe linezolid-induced haematological toxicity. The linezolid-containing 9-month regimen must not be offered to patients with a pretreatment serum Hb below 8 g/dL that cannot be rapidly corrected (i.e. with a blood transfusion) before starting MDR/RR-TB treatment. Similarly, due to the morbidity associated with severe neutropenia and thrombocytopenia, the linezolid-containing 9-month regimen is not suitable in patients with neutrophils below 0.75 × 10⁹ /L or platelets below 150 × 10⁹ /L before starting treatment.
5.2 The modified 9-month regimens for MDR/RR-TB
This section refers to the new modified 9-month treatment regimens for MDR/RR-TB developed in the endTB trial. The recommendations in the WHO consolidated guidelines on tuberculosis. Module 4: Treatment and care (1) state:
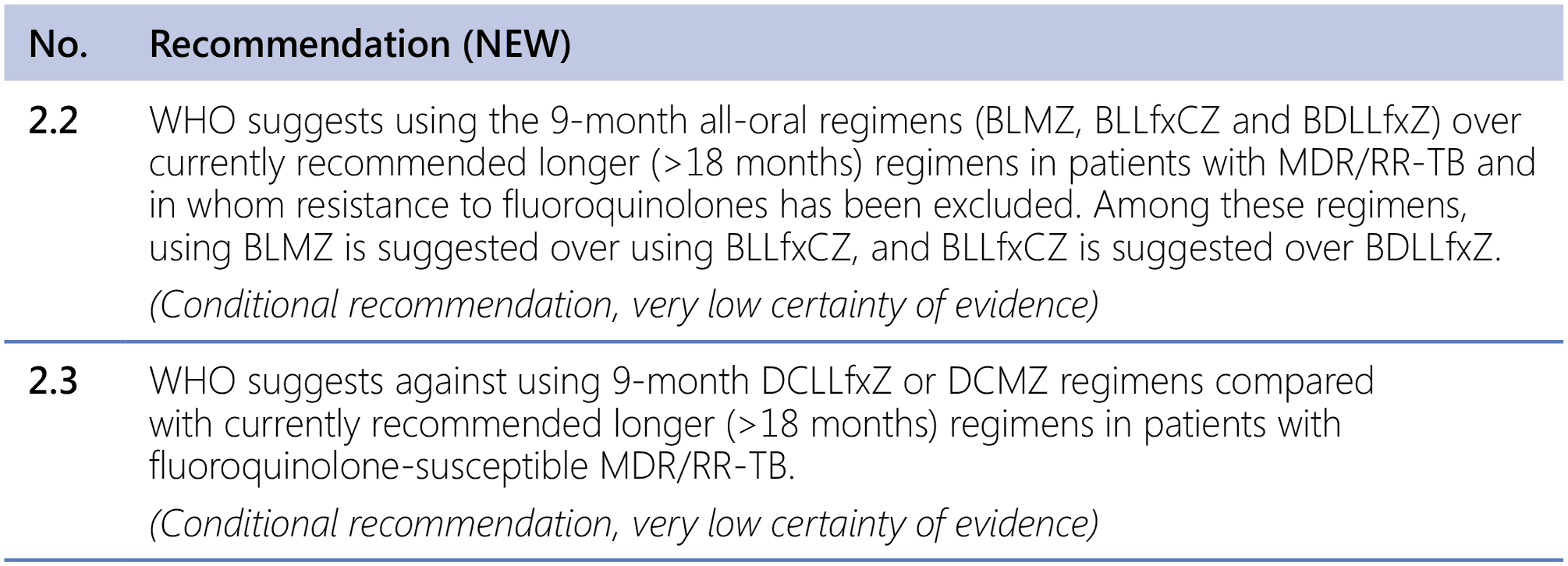
Remarks
- The recommended modified 9-month all-oral regimens comprise bedaquiline, linezolid and pyrazinamide in different combinations with levofloxacin/moxifloxacin, clofazimine and delamanid.
- This recommendation applies to the following:
- People with MDR/RR-TB and in whom resistance to fluoroquinolones has been excluded.
- People with diagnosed pulmonary TB, including children, adolescents, PLHIV, and pregnant and breastfeeding women.
- People with extensive TB disease and all forms of extrapulmonary TB except for TB involving the CNS, osteoarticular TB or disseminated forms of TB with multiorgan involvement.
- People with MDR/RR-TB and less than 1 month of previous exposure to any of the component medicines of the regimen (apart from pyrazinamide and fluoroquinolones). When exposure is greater than 1 month, these patients may still receive one of the regimens if resistance to the specific medicines with such exposure has been ruled out.
- Children and adolescents who do not have bacteriological confirmation of TB or resistance patterns but do have a high likelihood of MDR/RR-TB (based on clinical signs and symptoms of TB, in combination with a history of contact with a patient with confirmed MDR/RR-TB).
The recommendations for the three new modified 9-month all-oral regimens for MDR/RR-TB are primarily based on findings from the endTB trial, a non-blinded, RCT with five experimental arms compared with a control group. The control arm of the endTB trial used the longer WHO-recommended regimen, because enrolment occurred before the 2022 WHO recommendation establishing BPaLM as the preferred regimen for MDR/RR-TB. After reviewing the trial data, the GDG has determined that the BLMZ, BLLfxCZ and BDLLfxZ regimens are preferred over the longer regimens currently in use. The recommended four- and five-drug regimens include different combinations coming from a set of seven TB drugs commonly used in DR-TB treatment: bedaquiline (B), levofloxacin (Lfx), moxifloxacin (M), linezolid (L), clofazimine (C), delamanid (D) and pyrazinamide (Z). These regimens are specifically recommended for patients with MDR/RR-TB who do not have FQ resistance and they apply to diverse groups as outlined in the recommendation remarks (see Section 5.2.1 below).
All three regimens demonstrated high efficacy, with week 104 treatment outcomes showing favourable results in 89% of patients on BLMZ (95% CI: 82–94%), 89% on BLLfxCZ (95% CI: 81–94%) and 85% on BDLLfxCZ (78–91%).
Among the modified 9-month regimens, BLMZ is preferred over BLLfxCZ, and BLLfxCZ is preferred over BDLLfxZ. The WHO GDG made this ranking based on an evaluation of all evidence and judgements made for individual regimens, together with multiple comparisons of three recommended regimens based on the following six decision criteria: balance of effects, resources required, cost–effectiveness, equity, acceptability and feasibility (1). The rationale for the ranking can be summarized as follows:
- BLMZ was preferred over BLLfxCZ and BDLLfxZ:
- BLMZ appeared preferable in terms of the balance of health effects compared with both BLLfxCZ and BDLLfxZ;
- BLMZ has the lowest cost and pill burden, and appeared either preferable or equivalent for all other decision criteria; and
- BLMZ was therefore deemed to be the preferred regimen among the three.
- BLLfxCZ, was preferred over BDLLfxZ:
- BLLfxCZ, compared with BDLLfxZ, was deemed to have a similar but slightly preferable balance of health effects;
- BLLfxCZ also has a significantly lower cost and a lower pill burden than BDLLfxZ;
- the much greater cost of BDLLfxZ was judged as being likely to have negative effects on equity, acceptability and feasibility; and
- BLLfxCZ was therefore deemed to be preferrable to BDLLfxZ.
Although all three regimens showed a similar proportion of participants experiencing Grade 3 or higher AEs, BLMZ consists of only four drugs, which may result in a lower pill burden and fewer side-effects. BLLfxCZ includes five drugs, with levofloxacin offering a better side-effect profile regarding QT prolongation than moxifloxacin; however, the inclusion of clofazimine, known for causing significant QT prolongation and other AEs, is a drawback. BDLLfxZ also contains five drugs, but while delamanid has a favourable side-effect profile, its high cost makes this regimen less favourable.
Two of the five regimens used in the endTB trial, DCLLfxZ and DCMZ, underperformed compared with the others, leading to a recommendation against their use. Specifically, the DCLLfxZ and DCMZ regimens exhibited much higher rates of failure and acquired drug resistance than the longer regimen and the three recommended modified 9-month regimens. The absence of bedaquiline, a potent bactericidal drug, may explain their reduced efficacy. Until further evidence clarifies their potential role, these regimens should not be used.
Additionally, the GDG had access to data from operational research cohorts that used various four- and five-drug 9-month regimens under programmatic conditions. However, these regimens often deviated from the three recommended regimens (BLMZ, BLLfxCZ and BDLLfxZ). Specifically, many operational research cohort regimens excluded pyrazinamide and frequently included cycloserine. Due to the limitations of evidence – primarily derived from observational studies that lack direct comparisons and are challenging to align with clinical trial data – the GDG was unable to directly compare these alternative 9-month regimens with the newly recommended modified 9-month regimens. Nevertheless, evidence from operational research studies, including one conducted across more than 10 eastern European countries using a regimen similar to BCLLfxZ (with cycloserine replacing pyrazinamide), provided valuable reassurance about the feasibility of implementing such regimens. It is expected that this evidence will support and expedite the adoption of the recommended modified 9-month regimens, particularly in countries that participated in the operational research studies.
The role of the modified 9-month regimens within the broader range of WHO-approved treatments for MDR/RR-TB is discussed in detail in Section 3.3.
5.2.1 Eligibility
In settings where the 6-month MDR/RR-TB regimen is not yet available, or implementation of the regimen is not yet feasible, or for patients who are not eligible, selected patients with MDR/RR-TB may benefit from a 9-month all-oral regimen or modified 9-month regimens. The modified 9-month regimens may be offered to the following groups:
- People with MDR/RR-TB in whom resistance to FQ has been excluded.
- People with diagnosed pulmonary TB, including children, adolescents, PLHIV, and pregnant and breastfeeding women.
- People with extensive TB disease and all forms of extrapulmonary TB except for TB involving the CNS, or osteoarticular or disseminated forms of TB with multiorgan involvement.
- People with MDR/RR-TB and less than 1 month of previous exposure to any of the component medicines of the regimen (apart from pyrazinamide). When exposure is greater than 1 month, these patients may still receive one of the regimens if resistance to the specific medicines with such exposure has been ruled out.
- Children and adolescents who do not have bacteriological confirmation of TB or resistance patterns but who do have a high likelihood of MDR/RR-TB (based on clinical signs and symptoms of TB, in combination with a history of contact with a patient with confirmed MDR/RR-TB).
The endTB trial specifically targeted patients with MDR/RR-TB who were FQ-susceptible. Before initiating any MDR/RR-TB regimen, it is essential to conduct a WHO-recommended rapid molecular diagnostic test to detect FQ resistance. In areas where testing is not feasible, it is advisable to opt for one of the 6-month regimens recommended for MDR/RR-TB regardless of FQ resistance.
Patients with extensive TB disease respond well to the three modified 9-month regimens. Other key subgroups of patients (e.g. children, pregnant women, PLHIV and people with other comorbidities such as diabetes and viral hepatitis) are discussed in Section 5.2.3 below.
DDIs are common with many of the medications used in MDR-TB regimens, especially those involving bedaquiline. Details of interactions for drugs used in all WHO-recommended regimens is provided in Annex 1 and Annex 2. In each of the above DDI situations, if the clinician determines that the potential benefits outweigh the risks (considering alternative treatment options), treatment may proceed with caution.
Patients who have resistance to bedaquiline or linezolid, regardless of FQ susceptibility, are not eligible for the 6-month regimens or any of the 9-month regimens. In these cases, an individualized longer treatment regimen (see Section 6) may be the only viable option.
5.2.2 Composition, dosing and duration of the regimen
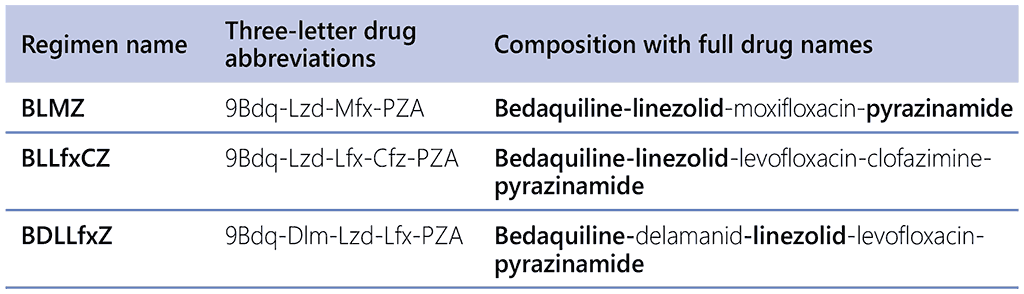
The short names of the regimens with one-letter abbreviations for the drugs, the three-letter drug abbreviations, and the compositions of the modified 9-month regimens are given in Table 2.5.2. All the modified 9-month regimens have bedaquiline, linezolid and pyrazinamide as a core, with one or two additional drugs.
Table 2.5.2. The composition of the three modified 9-month regimens
Dosing and frequency of component medicines
The dosing for drugs in the modified 9-month regimens follows Annex 4 for weight-based dosing of medicines used in MDR-TB regimens, for adults and children.²² The dosing for linezolid and bedaquiline in these regimens:
- Bedaquiline is dosed at 400 mg (four 100 mg tablets) daily for the first 2 weeks, followed by 200 mg (two 100 mg tablets) three times a week (e.g. Monday, Wednesday and Friday) in adults for the full 9-month period. An alternative dosing regimen for bedaquiline involves taking 200 mg daily for the first 8 weeks, followed by 100 mg daily for the full 9-month period.
- Linezolid is given at 600 mg once daily for 16 weeks then reduced to 300 mg once daily or 600 mg three times a week until the end of treatment for an adult.
In the endTB trial, participants were randomized patients to one of these two reduced-dose strategies for linezolid. The was no significant difference in AEs or efficacy between the two dosing strategies, although the relatively small sample size limited the ability to draw definitive conclusions. Therefore, either linezolid dose reduction strategy is acceptable. Linezolid dosing may also be reduced or even permanently discontinued earlier if toxicity occurs before 16 weeks but ideally not earlier than 9 weeks, provided there is a good clinical response demonstrated by culture conversion, resolution of symptoms or no radiological worsening. In all three modified 9-month regimens dose reduction of linezolid was a standard practice, which differs slightly from linezolid dosing used in other treatment regimens.
Duration
In the modified 9-month regimens, all drugs are typically administered for the entire 9-month duration, and treatment extensions beyond this period for slow clinical responses are not routinely recommended.
If the regimen is suspended owing to clinical AEs or laboratory abnormalities, treatment should resume as soon as the patient’s condition allows. Missed doses of more than 7 days and less than a month should be added up following standard case management.
For the modified 9-month regimens, a reasonable requirement is for patients to complete all doses within an 11-month timeframe, although this should be assessed on a case-by-case basis. Missing doses early in treatment is likely to have a greater negative impact on outcomes than those missed later in the regimen. For example, missing doses early in treatment, even if for less than 30 days, may require immediate repeat DST for all regimen drugs to assess potential resistance and determine whether a new treatment regimen is necessary.
Treatment discontinuation
The clinical assessment and bacteriological response at month 4 are routinely used to evaluate whether the patient is responding to treatment. If there is no improvement by month 4 (either clinically or if the culture remains positive at month 4 and beyond), investigations for a possible treatment failure or acquired drug resistance should be carried out. If there is no improvement by month 6 and beyond, either clinically or if the culture remains positive, consideration should be given to stopping the regimen and transitioning to a new, typically longer (18-month) regimen. The new regimen composition should be based on DST results.
If FQ resistance develops during treatment with the modified 9-month regimen, or if resistance develops to any other drug in the regimen (except pyrazinamide) during treatment, treatment should be declared a failure. In such cases, a new, longer regimen tailored to the patient’s DST profile should be initiated promptly.
Discontinuation of either pyrazinamide or linezolid owing to AEs may be considered and the regimen continued with the remaining drugs. However, if more than a single drug needs to be discontinued, the regimen should be stopped and an alternative treatment started.
5.2.3 Key subgroups
Various subgroup analyses were available to the GDG in considering the generalizability of the evidence on the modified 9-month regimens: age, PLHIV, previous TB treatments and viral hepatitis antibody status. The GDG also considered the eligibility criteria as stipulated by the endTB trial. The resulting considerations when prescribing the modified 9-month regimen are summarized below.
Children
Children were not included in the endTB trial. However, the medications that make up the modified 9-month all-oral regimens have been used for many years in MDR/RR-TB regimens for both adults and children, often in similar combinations. The associated adverse drug reactions are well documented (see Annexes 1 and 2), and weight-based dosing schedules for these drugs are provided in Annex 4. The BLMZ regimen is the preferred modified 9-month regimen because of the low pill burden and available child-friendly formulations. In situations where the child-friendly formulations are unavailable, practical guidance on adjusting adult formulations for children is provided (68), ensuring that lack of paediatric-specific formulations does not hinder access to treatment.
AEs appear to be less frequent in children than in adults; however, close monitoring is still warranted in children, regardless of the regimen. In the modified 9-month regimens, linezolid is the most challenging drug to monitor for side-effects in children, particularly for haematological side-effects, which requires repeated testing during the treatment. Additionally, detecting signs of peripheral neuropathy, as well as changes in visual acuity and colour vision, can be more challenging in younger children than in older children and adults. Despite these challenges, it is crucial to rigorously monitor for these common AEs associated with linezolid. Further details are given in Annex 2.
Pregnancy
Data on the optimal management of MDR/RR-TB in pregnancy are limited. The endTB trial excluded pregnant patients before initiation of the regimen. Other studies provide evidence that MDR/RR-TB can be managed in pregnancy (69) but caution is warranted for many drugs and regimens. Given that the BLMZ modified 9-month regimen contains only four drugs and the individual drugs have been used in pregnancy, this regimen is the preferred option among the modified 9-month regimens.
Whenever a pregnant woman is placed on an MDR/RR-TB regimen, it is important to ensure close monitoring on safety and efficacy, which is crucial for improving care for pregnant women with MDR/RR-TB (70).
Breastfeeding women
For reasons similar to those seen in pregnancy, BLMZ is a good regimen choice in breastfeeding women. This regimen offers four medicines that do have some history of being used in breastfeeding women, however, more evidence is needed.
For various reasons, breastfeeding is still the ideal strategy for women and their infants. This is true for women with MDR/RR-TB on treatment as well. Little is known about the safety of most second-line medications during breastfeeding, although the doses of medications that can be passed to the infant in breast milk are usually quite low. Appropriate infection control measures should be followed during breastfeeding. Mothers who have resources, or can be provided resources and training, for infant formula, clean water, fuel for boiling water and a heating device may choose to use infant formula.
People living with HIV
The three modified 9-month regimens evaluated in the endTB trial suggested good outcomes for PLHIV, and the subgroup analyses did not suggest any effect modification in this sub-population, compared to the overall trial population. However, the limited number of PLHIV across all treatment arms restricts the ability to make definitive comparisons or conclusions about outcomes in this population (71).
The DDIs and overlapping toxicities with TB drugs and ART described in Annex 1 and Annex 2 apply to the modified 9-month regimens. More details on the management of MDR/RR-TB patients and HIV are described in Section 8.3.
Pulmonary TB
The endTB trial enrolled patients with all forms of pulmonary TB, ranging from mild disease to severe cases with extensive fibrosis and cavitation. This inclusive approach ensured a comprehensive evaluation of the regimens across a spectrum of disease severity. In the endTB trial, patients receiving the modified 9-month regimens had lower overall success rates if they had cavities or high-grade sputum smears; however, even in these subgroups, the success rates appeared better than in the control arm. These results underscore the importance of timely diagnosis and treatment initiation to minimize disease progression and improve patient prognosis. The modified 9-month regimens can be used in all forms of pulmonary TB.
Extrapulmonary TB
The endTB trial did not have exclusion criteria for extrapulmonary TB, but it enrolled few patients with extrapulmonary TB and no patients with severe forms of extrapulmonary TB. As a result, the efficacy of the modified 9-month regimens in treating severe forms of extrapulmonary TB (e.g. CNS TB, osteoarticular TB or disseminated TB) remains unproven.
Patients with comorbidities other than HIV
Patients with diabetes mellitus
The modified 9-month regimens can be used in people with diabetes; however, treatment of DR-TB is more complicated in this population:
- diabetes is a risk factor for renal disease and peripheral neuropathy;
- close monitoring for peripheral neuropathy is warranted in people with diabetes on linezolid, with a low threshold for lowering the dose or stopping the drug if peripheral neuropathy develops or exacerbates while on treatment; and
- treatment of diabetes with normalization of the HbA1C should occur concurrently with MDR-TB treatment.
Patients with hepatic dysfunction
Owing to the hepatotoxicity associated with pyrazinamide, alternative regimens may be more appropriate for patients with moderate to advanced liver disease. In the endTB trial, the modified 9-month regimens were not initiated in patients with ALT levels exceeding three times the upper limit of normal.
For hepatitis C infection, direct-acting antivirals (DAAs) are generally well tolerated when co-administered with MDR/RR-TB treatment (72). Experience with DAAs alongside the modified 9-month regimens is limited; however, they can be safely used together in patients without advanced hepatic dysfunction or significant liver damage.
Patients with chronic renal insufficiency
Pyrazinamide is primarily excreted through the kidneys, and its metabolites can accumulate in patients with chronic renal insufficiency, increasing the risk of toxicity, including hyperuricemia and gout. In patients with impaired renal function, dose adjustment is essential to minimize these risks while maintaining therapeutic efficacy (Annex 1). All the other medications in the modified 9-month regimen are metabolized by the liver and do not need to be dose adjusted in chronic renal insufficiency.
Patients with anaemia
To manage anaemia in patients on the modified 9-month regimens (these regimens include linezolid, which causes myelosuppression), it is essential to assess and address both TB-related anaemia and potential linezolid-induced myelosuppression. In general, patients with a pretreatment Hb level below 8 g/dL should not start the regimen unless the anaemia can be rapidly corrected; for example, with blood transfusion, erythropoietin or a combination of both (Annex 2 has additional details).
Nutritional deficiencies, such as iron deficiency, may improve as TB symptoms resolve with effective treatment, although oral iron supplementation is often poorly tolerated and not immediately effective. Myelosuppression can be life threatening; thus, careful monitoring of Hb, neutrophil and platelet levels is critical during treatment. In most cases, myelosuppression is reversible with temporary suspension of linezolid. If Hb levels fall to 8–10.5 g/dL, a reduced dose or temporary suspension should be considered. If Hb drops below 8 g/dL during the first 4 months of therapy because of linezolid toxicity, linezolid should be temporarily discontinued; if Hb does not recover, the linezolid may need to be permanently stopped. If the patient receives linezolid for less than 4 months, the regimen may need to be modified or a new regimen given. Temporary blood transfusions may allow the patient to initiate or continue treatment while the underlying TB is controlled, because anaemia often improves as the disease resolves. However, ongoing exposure to linezolid can exacerbate anaemia, and transfusions alone may not counteract its effects. Monitoring and timely management are key to balancing treatment efficacy with patient safety.
5.2.4 Implementation considerations
DST considerations
The endTB trial specifically targeted patients with MDR/RR-TB who were fluoroquinolone-susceptible. An mWRD for FQ resistance should be performed before initiating treatment. If testing is unavailable, one of the 6-month regimens outlined in Section 4 should be considered, provided the patient meets eligibility criteria.
Patients who have resistance to bedaquiline or linezolid, regardless of fluoroquinolone susceptibility, are not eligible for the 6-month regimens or any of the 9-month regimens. In these cases, an individualized longer treatment regimen (see Section 6) may be the only viable option.
Extent of the disease
Patients with extensive TB disease responded well to the three modified 9-month regimens.
Drug-drug interactions (DDIs)
DDIs are common with many of the medications used in MDR-TB regimens, especially those involving bedaquiline. Annex 1 and Annex 2 provide details of drug interactions for all WHO-recommended regimens. In each of the above DDI situations, if the clinician determines that the potential benefits outweigh the risks (considering alternative treatment options), treatment may proceed with caution.
Role of pyrazinamide in the modified 9-month regimen
An unpublished analysis of the endTB trial is ongoing to evaluate further the role of pyrazinamide within the modified 9-month regimens. The highlights from this assessment are outlined below (73).
Performance based on pyrazinamide sensitivity and resistance
In the endTB trial, patients receiving the modified 9-month regimens had lower overall success rates if they had baseline pyrazinamide resistance; however, even in this subgroup, the success rates appeared to be better than in the control arm, although the difference was not statistically significant. These data suggest that pyrazinamide sensitivity offers a marginal advantage but that pyrazinamide resistance does not severely impact the overall efficacy of the regimens – the modified 9-month regimens remain effective even in cases of pyrazinamide resistance.
Hepatotoxicity and discontinuation of pyrazinamide
Screening for elevation of liver enzymes was performed monthly throughout treatment, regardless of symptoms. In patients with risk factors for liver toxicity (e.g. alcohol abuse disorder or active viral hepatitis infection) liver enzyme testing should be conducted at least every 2 weeks during the first 2 months of treatment. If no signs or symptoms of hepatotoxicity develop, this should change to monthly liver enzyme testing for the remainder of the regimen. Elevation in liver enzymes, with or without accompanying symptoms, occurred frequently during treatment. Grade 3 hepatotoxicity was defined in the trial as ALT (SGOT) or AST (SGOP) levels greater than five times but less than or equal to 20 times the upper limit of normal. Transient Grade 3 or higher hepatotoxicity occurred in 18.3% of patients in BLMZ, 15.6% in BLLfxCZ, and 8.7% in BDLLfxZ in the safety population of the endTB trial; these differences were not statistically different across the regimens.
During the trial, suspension of pyrazinamide was recommended when liver enzyme levels exceeded five times the upper limit of normal. The drug was permanently discontinued in an average of 17% of patients, with no significant differences in the 73-week modified intention-to-treat treatment outcomes among the three regimens. Most of the patients receiving the modified 9-month regimen received 39 weeks of pyrazinamide, and patients who permanently discontinued pyrazinamide received the drug for between 85 and 112 days, again with minimal variation between the regimens.
SoC for pyrazinamide management in the modified 9-month regimens
The following are suggested practices for managing pyrazinamide in the modified 9-month regimens:
- LFTs:
- ALT and AST should be tested at baseline. More frequent testing should be considered in the presence of individual risk factors for liver toxicity.
- Pyrazinamide DST:
- If DST is available, it should be used at baseline; however, regimens may still be used in the absence of pyrazinamide resistance testing or while waiting for results.
- If the isolate is susceptible to pyrazinamide, the regimen should be continued, with pyrazinamide only discontinued if toxicity occurs.
- If resistance to pyrazinamide is confirmed, the drug can be discontinued. This guidance, provided by the GDG, recognizes the potential for limited retained activity owing to possible synergy between bedaquiline and pyrazinamide (74), even in the presence of pyrazinamide resistance. However, this synergy is likely to be modest and must be carefully weighed against pyrazinamide’s toxicity profile.
- In all cases, whether pyrazinamide-resistant or pyrazinamide-sensitive, standard practices on treatment outcome assessment should be followed regarding when to switch the regimen if the patient is not responding well to treatment (see Section 9.7).
- No pyrazinamide DST available:
- In the absence of pyrazinamide DST, decisions regarding temporary suspension, permanent discontinuation or switching to regimens that do not employ pyrazinamide (6-month regimens or the longer regimen) should be based on the severity of pyrazinamide-induced hepatotoxicity. Principles for managing drug-induced hepatotoxicity are detailed in Annex 2.
In summary, the similarity in treatment outcomes between pyrazinamide-resistant and pyrazinamide-sensitive strains across all three modified regimens underscores the robustness of the modified 9-month regimens. Although pyrazinamide sensitivity appears to provide an advantage, the resistance does not significantly hinder treatment success. Thus, even though pyrazinamide contributes positively to treatment, it does not appear to be the critical driver of success in these regimens. Use of pyrazinamide requires careful monitoring. In patients with multiple risk factors for hepatotoxicity or advanced liver disease (with or without pyrazinamide resistance), more frequent liver enzyme monitoring or use of alternative regimens may be more appropriate. Regular LFTs and sound clinical judgement are critical to optimizing treatment outcomes while minimizing the risk of serious AEs.
Cost of the modified 9-month regimen
The price of medicines for the two modified 9-month regimens that do not include delamanid is relatively low (Table 2.5.3). However, the overall operational costs of implementing a 9-month treatment regimen may be higher than the 6-month regimens outlined in Section 4, primarily due to the longer duration of care. Additionally, the higher rates of hepatotoxicity associated with the 9-month regimens could result in increased costs for monitoring and managing liver-related complications. Apart from hepatotoxicity, the safety profile of the modified 9-month regimens is comparable to that of the 6-month regimens, with costs likely to be similar to those for managing other AEs.
Table 2.5.3. Prices and pill burden of the modified 9-month regimens
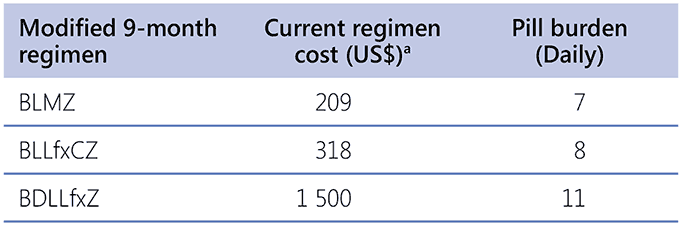
a The lowest available prices from the Global Drug Facility, effective from 1 January 2025, which are subject to change (38).
Care and support
To optimize treatment outcomes, all TB patients should receive a patient-centred approach combined with a comprehensive package of care and support. The excellent results of the modified 9-month regimens in the endTB trial may be attributed, in part, to the robust patient-centred approach that provided significant support throughout treatment and beyond. This support extended far beyond simply providing medication and addressed adherence, side-effect management and the overall well-being of patients. To replicate the same high rate of treatment success, similar support packages should be used for all DR-TB regimens.
The comprehensive, patient-centred care and support provided in the endTB trial was critical for achieving successful treatment outcomes. This approach should be considered essential for the implementation of all TB regimens. The recommended comprehensive support package to be used with modified 9-month regimens includes the following:
- Patient education: Patients should be educated through one-on-one counselling and printed materials to ensure that they understand their treatment, potential side-effects and the importance of adherence.
- AE monitoring and management: Regular monitoring for side-effects is a cornerstone of implementation of the modified 9-month regimens. Patients should be routinely screened for AEs, and medical staff trained to manage side-effects promptly and effectively.
- Treatment support: Patients should receive daily supervision of medication intake, either in-person or via digital tools where applicable, to maintain adherence and treatment fidelity. Treatment support is encouraged for the modified 9-month regimens.
- Counselling and psychosocial support: Patients should receive tailored counselling on treatment adherence, side-effect management and lifestyle adjustments. Psychosocial support can address emotional and social challenges associated with MDR-TB. Peer groups and community health workers can play a vital role in providing education, encouragement and adherence support.
- Nutritional support: Recognizing the impact of undernutrition on treatment outcomes, nutritional assessments and counselling, followed by nutritional interventions recommended by WHO are essential; this will ensure adequate nutrition during the treatment period, helping to improve recovery rates.
- Financial assistance: Providing some patients with financial support for transportation, food or other treatment-related expenses can reduce barriers to care. By alleviating financial burdens, patients can better focus on their recovery.
19 This includes exposure to FQs as either treatment or prevention of MDR/RR-TB.
20 See Definitions section.
21 See Definitions section.
22 Slightly different dosing was used in the endTB trial for patients with lower body weight. Levofloxacin was dosed at 750 mg per day (versus 500 mg) for the >24–30 kg category, and pyrazinamide was dosed at 800 mg for >24–30 kg and >30–35 kg, and 1200 mg for >35–40 kg. These differences are minor, and programmes may adopt either the endTB trial dosing or the recommendations in Annex 1 and Annex 4.

 Обратная связь
Обратная связь