Перекрёстные ссылки книги для ПЛАТФОРМА ВОЗ ПО ОБМЕНУ ЗНАНИЯМИ О ТБ
Treatment of DS-TB poses special issues in some subgroups of patients; in particular, those with diabetes, pregnant women, people aged over 65 years, and those with chronic kidney or liver disease.
8.1 Diabetes
Diabetes is a common condition, particularly in some countries, where up to 30–40% of TB patients are affected. The population attributable fraction of diabetes as a risk factor for TB is more than 10% in all WHO regions, except for Africa and the Western Pacific (4). Diabetes was estimated to account for more than 10% of global TB deaths among HIV-negative individuals (61).
Hyperglycaemia induces abnormalities in both the innate and adaptive immune response to M. tuberculosis, and diabetes increases the risk (twofold to fourfold) that TB infection will progress to disease; also, the response to treatment is often worse in those with diabetes. Among the mechanisms involved, bacterial recognition and phagocytosis are less effective in diabetes, with impairment of antigen-presenting cell recruitment and delay in activating the cellular immune response (62). Clinically, this translates into an increased proportion of sputum smear positive patients, with more extensive pulmonary disease bilaterally, larger number of cavities and lymph node enlargement, and “atypical” findings of lower lobe lesions (especially in patients with poor glycaemic control). People with diabetes also suffer an increased rate of failure and death, and a higher risk of relapse (62).
Diabetes has a negative effect on the pharmacology of some anti-TB drugs (e.g. rifampicin), with higher risk of development of drug resistance (62). Rifampicin is a potent hepatic enzyme inducer, increasing the hepatic metabolism of sulphonyl urea derivatives and therefore lowering their plasma levels. No effect of rifampicin is known on the exposure of glucagon-like peptide-1 receptor agonists and only a slight effect on dipeptidyl peptidase-4 inhibitors. Although metformin is not metabolized by the P450 enzymes system, its hypoglycaemic effect may be increased by rifampicin, enhancing the expression of organic cation transporter and the hepatic uptake of metformin. Because insulin is not metabolized, no pharmacokinetic interactions with anti-TB drugs occur; therefore, some authors have recommended that it be used at the beginning of TB treatment, to achieve faster bacteriological sputum conversion and prevent DDIs (62).
A higher proportion and sometimes a greater severity of AEs has been described in TB patients with diabetes (e.g. peripheral neuropathy due to isoniazid and ocular neuropathy due to ethambutol) (62).
There is evidence that the problems described above reduce when diabetes is well controlled. Therefore, adequate control of diabetes, and collaboration between TB and diabetes services, are important, particularly in countries with a high prevalence of diabetes.
Implementation considerations
- Although the drugs used to treat DS-TB are generally well tolerated and are unlikely to cause serious AEs among people with diabetes, treatment monitoring is important to ensure rapid notification and prompt management of any side-effects that eventually appear.
- Management of these patients involves a multidisciplinary approach, in view of the additional need to control diabetes and the potential need to adjust drug dosing. A national or subnational body supporting the management of people with difficult-to-treat TB (i.e. a consilium) may be of help in specific cases (63).
- Supporting adherence is an important management component when treating people with DS-TB and diabetes. Therefore, collaboration with partners in the community, including family members, carers, health care workers and welfare workers, is essential.
- Coordination of NTPs with diabetes services may be relevant in countries where TB is highly prevalent.
8.2 Pregnancy
Epidemiological information on TB in pregnancy is scarce. In the United Kingdom, women in early postpartum were twice as likely to develop TB as non-pregnant women (64).
A recent population study in Mozambique evaluated the prevalence of TB in pregnancy and found that it was similar to that of the general population, although it was higher in women living with HIV (65). The TB prevalence was 505 (95% CI: 242–926) per 100 000 pregnant women and 297 (95% CI: 61–865) per 100 000 postpartum women. Among pregnant women who were HIV-positive, TB prevalence was 1626 per 100 000 (95% CI: 782–2970) and among postpartum women who were HIV-positive, TB prevalence was 984 per 100 000 (95% CI: 203–2848).
In addition to the TB-related risks to the mother, TB during pregnancy has been associated with high perinatal mortality, small size for gestational age, preterm and low birth weight neonates (66). Maternal TB disease is associated with poorer neonatal outcomes, in part because of social deprivation and other factors that are associated with a higher risk of TB during pregnancy (67). Disseminated TB in the mother can cause congenital TB in the infant, but this is a rare condition (68). Diagnosis of TB is often delayed during pregnancy, because of its nonspecific symptoms and overlapping presentation with other infectious diseases. Adverse perinatal outcomes are even more pronounced in women with advanced disease, late diagnosis, and incomplete or irregular drug treatment. Many antenatal clinics are unprepared to diagnose TB (69). Because pregnancy is usually considered an exclusion criterion, there is a lack of data from clinical trials including this important category of patients. Standard treatment for DS-TB is considered safe in pregnancy and outweighs the grave risks posed by untreated TB. Measurement of liver function before the start of treatment is useful and, if the function is found to be abnormal, appropriate management is undertaken (70, 71). Core issues related to the management of treatment during pregnancy relate to the safety of the child before and after birth, considering both the risk of transmission (i.e. mother-to-child) and the potential teratogenic effect of anti-TB drugs.
Neonatal TB is most commonly due to inhalation of tubercle bacilli. As long as the mother has received at least 2 weeks of treatment for DS-TB, isolation of the infant is not required (72). This is particularly relevant because of the importance of breastfeeding for child health. Early diagnosis and treatment help to ensure the best possible outcome of TB in pregnancy for both mother and infant.
Pregnant women are usually treated with the standard 6-month 2HRZE/4HR regimen. Evidence on the use of the 4-month 2HPMZ/2HPM regimen during pregnancy is lacking (1). Experts have suggested using pyridoxine to complement the anti-TB regimen in pregnancy, because deficiency is more likely to occur than in the general population (73).
Implementation considerations
- The isolation needs of the mother should be reduced to the minimum necessary to prevent transmission to the child, to ensure that breastfeeding is not interrupted.
- Health education on the basics of infection control, with a special focus on personal protection and ventilation, is an important component of the management of treatment of DS-TB during pregnancy.
- Although the drugs used to treat DS-TB are generally well tolerated and are unlikely to cause AEs to the mother and the child, monitoring of AEs is important to ensure rapid notification and prompt management.
- Management of patients listed in this section (i.e. pregnant women and others) involves a multidisciplinary approach; a TB consilium to support the management of people with TB that is difficult to treat may be of help (63, 74).
- Coordination of the NTP with antenatal clinics and HIV services is important, to ensure rapid diagnosis and effective treatment of TB in pregnancy.
8.3 Older people
TB in older people is particularly relevant in countries with low incidence of TB in the WHO regions of the Americas and Europe, and is a growing problem in Asia because of the increasingly ageing population (4, 75). Outbreaks in nursing homes are frequently described, particularly in countries with a low incidence of TB (76, 77). The occurrence of TB among older people is also related to the higher prevalence of comorbidities (e.g. diabetes, chronic renal impairment and smoking) in this age group. The disability-adjusted life-years lost due to TB in patients aged over 65 years range from 8.2% in Europe to 18.7% in East and Central Asia (78).
The main challenges to successful treatment among older patients include poor drug tolerance, AEs and poor treatment adherence, all of which could potentially lead to unfavourable treatment outcomes.
Recent data from Japan on TB patients notified in 2017 indicate that the case-fatality rate increased with age, being 3.1% for those aged 0–64 years, 15.3% for those aged 65–74 years, 27.0% for those aged 75–84 years and finally 47.4% for those aged 85 years and over (44, 75). A study in Nigeria described lower sputum smear conversion after the intensive phase of treatment in patients aged over 60 years, although only extrapulmonary TB and HIV coinfection were significant predictors of a poorer outcomes (73).
Gastrointestinal upset and hepatitis are reported as the most frequent AEs in older people (79, 80). In Japan, in patients aged 80 years or more treated for DS-TB with the 6-month regimen, the prevalence of hepatitis was higher among those receiving treatment with isoniazid, rifampicin, pyrazinamide and ethambutol than among those receiving isoniazid, rifampicin and ethambutol, although treatment outcomes were similar in the two groups (81).
Clinical attention should be paid to older patients undergoing pyrazinamide treatment, to rapidly identify and manage any AEs that eventually appear. Guidelines from the American Thoracic Society consider the option of excluding pyrazinamide in patients aged over 80 years (44).
Ethambutol is excreted by the kidney. A low glomerular filtration rate (GFR) (i.e. <30 mL/minute−1) has a poor prognosis in the treatment of TB (82). In older people, the dose should be reduced according to the estimated GFR, but the time between doses should also be increased, to ensure that high blood levels of the drug do not persist (83).
Older individuals are likely to have several comorbidities and are therefore likely to be taking other medicines; hence, there is potential for DDIs (84). The interaction between the anticoagulant warfarin and rifampicin is especially problematic, and either heparin or a non-vitamin K oral anticoagulant are considerably safer. Other important interactions include those with statins, analgesics (e.g. celecoxib and losartan), oral antidiabetic medications, steroids, calcium channel blockers and theophyllines. When prescribing TB treatment in older people, it is always important to evaluate potential interactions among the different drugs prescribed to manage comorbidities (73).
Among older people, particular care is also necessary to ensure correct adherence to the prescribed treatment within a multidisciplinary and patient-centred approach (44, 85).
Implementation considerations
- Although the drugs used to treat DS-TB are generally well tolerated and are unlikely to cause AEs among older people, monitoring of AEs is important to ensure rapid notification and prompt management.
- Management of older people with TB involves a multidisciplinary approach, in view of the additional treatments that are often required to manage comorbidities and the potential need to adjust drug dosing. A TB consilium to support the management of people with TB that is difficult to treat may be of help (63).
- Supporting adherence, taking into account age-related physical and psychological disabilities, is an important management component when treating DS-TB in older people. Thus, collaboration with partners in the community, including family members, carers, health care workers and welfare workers, is essential.
- Coordination of NTPs with geriatric services may be relevant in countries where TB in older people is increasingly notified.
8.4 Chronic renal failure
Patients with chronic renal failure (CRF) have more frequent AEs and higher mortality rates than patients without CRF. This has been attributed to increased host susceptibility from the cellular immunosuppressive effects of CRF and to social determinants of health among those with CRF (86).
The severity of renal insufficiency is classified using creatinine clearance: it is mild when the rate of clearance is 60–120 mL/minute, moderate at 30–59 mL/minute, severe at 10–29 mL/minute and very severe at below 10 mL/minute. According to some experts, for patients with DS-TB on dialysis, a thrice-weekly dosing of pyrazinamide and ethambutol should be administered after the dialysis cycle (62, 86). Creatinine clearance is calculated using the following formula:
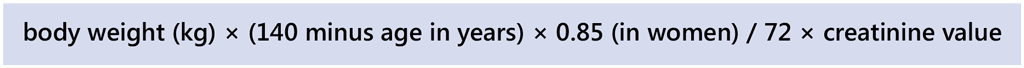
Dose adjustments in adults with creatinine clearance below 30 mL/minute are as follows (unless otherwise indicated):
- Pyrazinamide: 25–35 mg/kg per dose, three times per week after dialysis.
- Ethambutol: 15–25 mg/kg per dose, three times per week after dialysis.
- Rifapentine and moxifloxacin, which are both used in regimens for DS-TB, do not require renal dose adjustment (17, 87).
Experts recommend close monitoring of creatinine every week or every 2 weeks, and adequate hydration (71). Given the frequent occurrence of electrolyte disturbances in CRF, weekly monitoring of electrolytes is also recommended.
In the case of severe hypokalaemia, treatment is with intravenous potassium chloride (KCl) at 10 mEq/hour−1 (10 mEq of KCl will raise the serum potassium by 0.1 mEq/L−1). If the potassium level is low, checking the magnesium is recommended by experts; if this is not possible, empirical treatment with magnesium (i.e. magnesium gluconate at 1000 mg twice daily) should be considered in all cases of hypokalaemia. The use of spironolactone, 25 mg daily, is suggested in refractory cases (71).
Given the risk of QT prolongation (particularly due to moxifloxacin) and electrolyte imbalance, an ECG should be performed, taking into account that hypokalaemia may be refractory if the concurrent hypomagnesaemia is not corrected; the risk is higher if the intensive phase of treatment is prolonged for any reason; and electrolyte disturbances are reversible, although the disturbance might last weeks or months.
Implementation considerations
- Both the diagnosis of CRF and the treatment of TB in patients with CRF are challenging. There is little evidence to support evidence-based guidance for these patients.
- Given the complexities of the management of TB disease in patients with CRF, a close collaboration between infectious disease specialists, pulmonologists and nephrologists in this patient population is necessary. A TB consilium to support the management of people with TB that is difficult to treat may be of help (63, 74).
8.5 Chronic liver disease
Isoniazid, rifampicin or pyrazinamide may cause hepatotoxicity. In the management of TB in patients with chronic liver disease (CLD), experts recommend monitoring aminotransferases (i.e. alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) on a weekly basis initially, and fortnightly after the second month of treatment. In cases where aminotransferase are five or more times higher than the upper limit of normal (with or without symptoms), or three or more times higher in the presence of symptoms or jaundice (i.e. bilirubin >3 mg/dL−1), the treatment should immediately be withdrawn. The responsible drugs should be identified, and a sequential reintroduction implemented once enzyme levels have returned to normal. The drug reintroduction should be performed one drug at a time, starting with the drug considered to be the least hepatotoxic, as follows:
- when aminotransferases return to less than two times the upper limit of normal, rifampicin may be restarted with ethambutol;
- after 3–7 days, after checking aminotransferases, isoniazid may be reintroduced, with subsequent rechecking of liver enzymes; and
- if symptoms recur or aminotransferases increase again, the last drug added should be stopped and replaced with another from the list of the recommended drugs (71).
If the clinical pattern indicates cholestasis, rifampicin may be the responsible drug. If the patient has prolonged or severe hepatotoxicity but tolerates isoniazid and rifampicin, a rechallenge with pyrazinamide may be hazardous. In this situation, pyrazinamide may be permanently discontinued, with treatment eventually extended to 9 months (71). In patients with advanced CLD, coagulation factors should be carefully monitored (44, 88–90).
NTPs should consider stocking an extra supply of drugs to modify the HRZE regimen in the treatment of special situations such as CLD. Among the drugs that can be considered safe to use in patients with CLD are ethambutol and FQ (71). Given their important bactericidal and sterilizing action, where possible, isoniazid or rifampicin (or both) should be included (71).
A patient’s N-acetyltransferase (NAT) status affects their risk profile. Slow acetylators have a higher possibility of liver injury, so an isoniazid dose of 2.5–5 mg/kg/day may be adequate in such patients; in rapid acetylators, in contrast, the isoniazid dose may be increased to 7.5 mg/kg/day.
The Child–Turcotte–Pugh (CTP) score is based on albumin, bilirubin, prothrombin time/international normalized ratio (PT/INR), ascites and encephalopathy. The CTP score can be used as a predictor of tolerance to anti-TB drugs and the treatment outcome, as shown in Table 8.1 (91).
Table 1.8.1. CTP score parameters
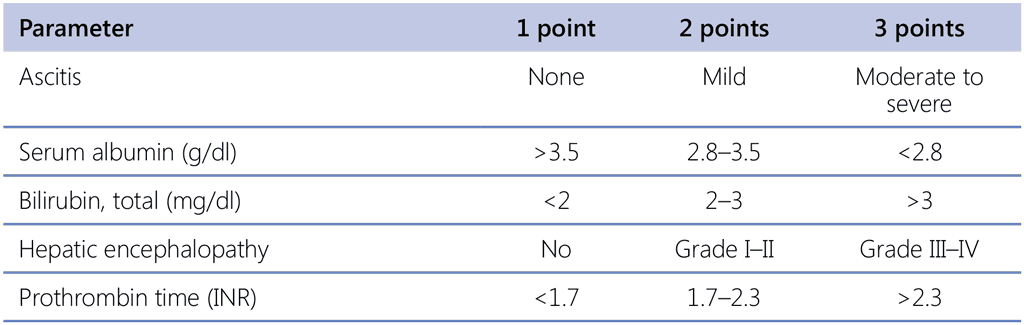
CTP: Child–Turcotte–Pugh; INR: international normalized ratio.
Table 1.8.2. Estimated survival at 1 and 2 years based on CTP

CTP: Child–Turcotte–Pugh.
In people with DS-TB with stable CLD (CTP ≤7), a treatment regimen that includes isoniazid, rifampicin and ethambutol is likely to be tolerated, with the exclusion of pyrazinamide (which is the most hepatotoxic drug in the 6-month regimen). Some experts suggest that, in this situation, the isoniazid and rifampicin continuation phase be prolonged to 7 months, after a 2-month intensive phase with the three drugs (91).
In patients with more severe CLD (CTP 8–10), it is advisable to use only one potentially hepatotoxic drug, preferably rifampicin; however, if CLD is very advanced (CTP ≥11), it is advisable to not use any hepatotoxic drug (71, 86). Some authors advise using a temporary liver-sparing regimen early in treatment to reduce bacillary load and transmission risks while waiting for transaminase levels to decrease.
When there is a need to design regimens for special situations, collaboration with clinicians who have specific experience in CLD and the support of an expert committee (e.g. TB consilium) are recommended (44, 63).
Implementation considerations
- In people with DS-TB and CLD, evaluation of the degree of impairment of the liver function is necessary, to design the best possible regimen that is sufficiently effective while not being aggressive for the liver. Given the clinical severity of these patients, collaboration with clinicians who have specific experience in CLD and the support of an expert committee (e.g. TB consilium) is recommended.
- The NTP should ensure a stock of individual formulations to manage patients with CLD who are unable to tolerate the standard recommended regimens.
- Treatment outcomes are often less favourable in patients with CLD than in patients without CLD.

 Обратная связь
Обратная связь