Перекрёстные ссылки книги для 1266
The 2021 WHO consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring provide recommendations based on rapidly evolving evidence of safety and efficacy and programmatic experience using DTG and low-dose EFV in pregnant women and people (including children and adolescents) with TB/HIV coinfection (78).
DTG is approved for use among children aged over 4 weeks and weighing more than 3 kg. A dispersible 10 mg formulation of DTG is available on the market. Among children for whom approved dosing of DTG is not available, RAL is considered an effective option and is approved for use from birth (with dose adjustments during TB treatment). RAL should be substituted with DTG as soon as it is available from 4 weeks of life.
DTG in combination with a NRTI backbone is recommended as the preferred first-line regimen for adolescents living with HIV and for infants and children with approved DTG dosing who are starting ART. EFV at low dose (400 mg) in combination with a NRTI backbone is recommended as the alternative first-line regimen for adolescents living with HIV starting ART (except in settings with pre-treatment HIV drug resistance to EFV or NVP over 10%). EFV 400 mg can be co-administered with rifampicin-containing TB treatment, with co-administration well tolerated and plasma concentrations maintained above the levels considered to be effective (181).
If DTG is not available, the preferred first-line ART regimen is a LPV/r-based regimen. RAL should be used only under special circumstances, such as in neonates. Beyond the neonatal period, infants and children should be transitioned to DTG as soon as possible.
Table 7.1 summarizes the preferred and alternative first-line ART regimens for neonates, children and adolescents on TB treatment (78).
Table 7.1. Preferred and alternative first-line antiretroviral therapy regimens for neonates, children and adolescents on TB treatment
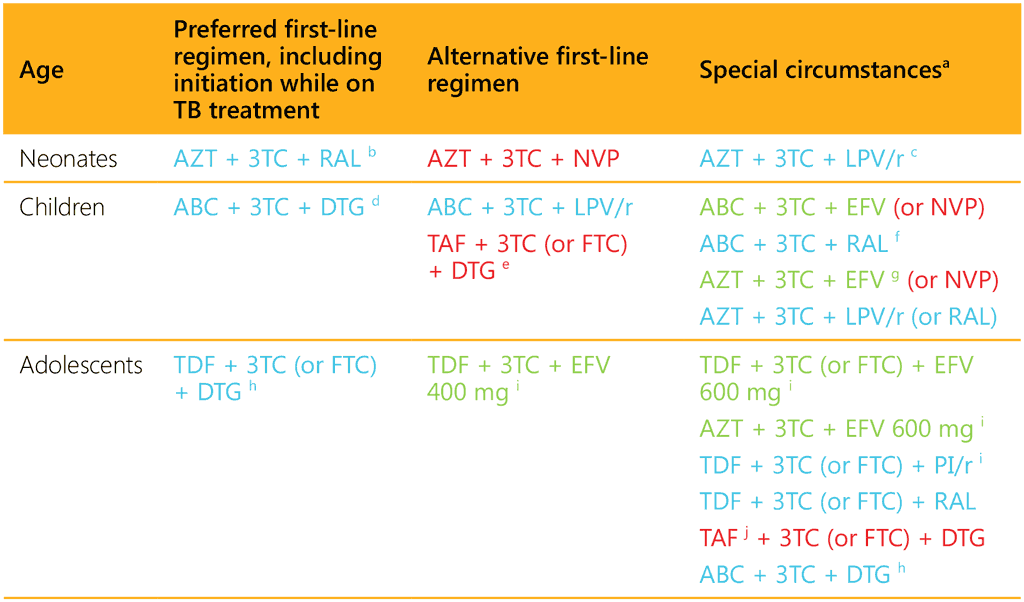
3TC: lamivudine; ABC: abacavir; AZT: zidovudine; DTG: dolutegravir; EFV: efavirenz; FTC: emtricitabine; LPV/r: lopinavir/ritonavir; NVP: nevirapine, PI/r: protease inhibitor boosted with ritonavir; RAL: raltegravir; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate.
No dose adjustment needed with TB treatment.
Dose adjustment needed with TB treatment.
Change of regimen needed with TB treatment.
For details on required adjustments in dose or regimen, see Table 7.2.
a Special circumstances include where it is not possible to give the preferred or alternative regimens, including toxicity, intolerance, inability to take the preferred or alternative medicine in the available formulation, and unavailability or stockouts.
b Neonates starting ART with a RAL-based regimen should transition to an LPV/r solid formulation as soon as possible.
c LPV/r syrup or granules can be used if starting after age 2 weeks.
d For age and weight groups with approved DTG dosing.
e For age and weight groups with approved TAF dosing.
f RAL should be used as an alternative regimen only if LPV/r solid formulations are not available.
g Efavirenz should not be used in children aged under 3 years.
h Effective contraception should be offered to adolescent girls of childbearing age or potential. DTG can be prescribed for adolescent girls of childbearing age or potential who wish to become pregnant or who are not otherwise using or accessing consistent and effective contraception if they have been fully informed of the potential increase in the risk of neural tube defects (at conception and until the end of the first trimester). If women identify pregnancy after the first trimester, DTG should be initiated or continued for the duration of the pregnancy.
i EFV-based ART should not be used in settings with national estimates of pre-treatment resistance to EFV of 10% or higher. DTG-based ART is preferred. If DTG is unavailable, a boosted PI-based regimen should be used. The choice of PI/r depends on programmatic characteristics.
j TAF may be considered for people with established osteoporosis or impaired kidney function. TAF has drug–drug interactions with rifamycin; as dose adjustments have not been established, concurrent use should be avoided.
DTG in combination with an optimized NRTI backbone may be used as a preferred second-line regimen for adolescents and children living with HIV with approved DTG dosing for whom non-DTG-based regimens are failing. Boosted protease inhibitors (PIs) in combination with an optimized NRTI backbone are recommended as a preferred second-line regimen for people living with HIV for whom DTG-based regimens are failing.

 Обратная связь
Обратная связь