Перекрёстные ссылки книги для 1266
4.1. Drug-susceptible TB
Research questions in a Population, Intervention, Comparator, Outcomes (PICO) format are listed below as they related to the recommendations retained in this policy consolidation.
4.1.1. Guideline update 2010
Recommendation 1. PICO question
Should new pulmonary TB patients be treated with the 6-month or the 2-month rifampicin regimen?
Recommendation 2. PICO question
When a country selects 2HRZE/4HR, should patients be treated daily or three times weekly during the intensive phase?
Recommendation 5. PICO question
In new pulmonary TB patients, how effective is extension of treatment for preventing failure or relapse?
Recommendation 8. PICO question
Should intermittent regimens be used for persons living with HIV? What should be the duration of TB treatment in people living with HIV?
4.1.2. Guideline update 2017
Recommendation 3. PICO questions
Does intermittent dosing in the intensive phase have outcomes similar to daily dosing in the intensive phase for treatment of drug-susceptible pulmonary tuberculosis?
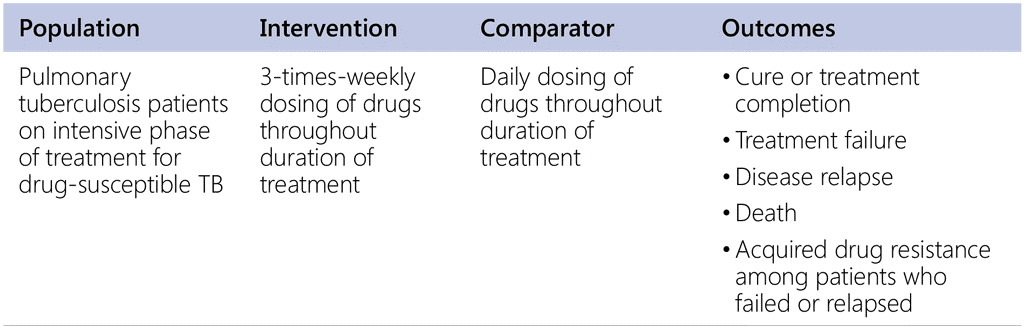
Does intermittent dosing in the continuation phase have outcomes similar to daily dosing in the continuation phase in patients with drug-susceptible pulmonary tuberculosis patients?
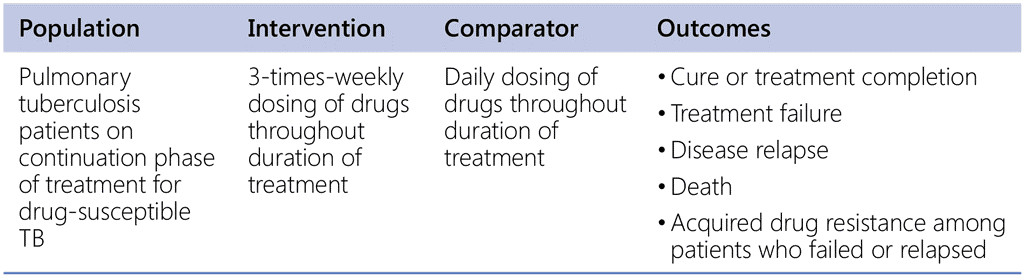
Recommendation 4. PICO question
In patients with active TB, is the use of fixed-dose combination (FDC) formulations as effective as the use of separate drug formulations?
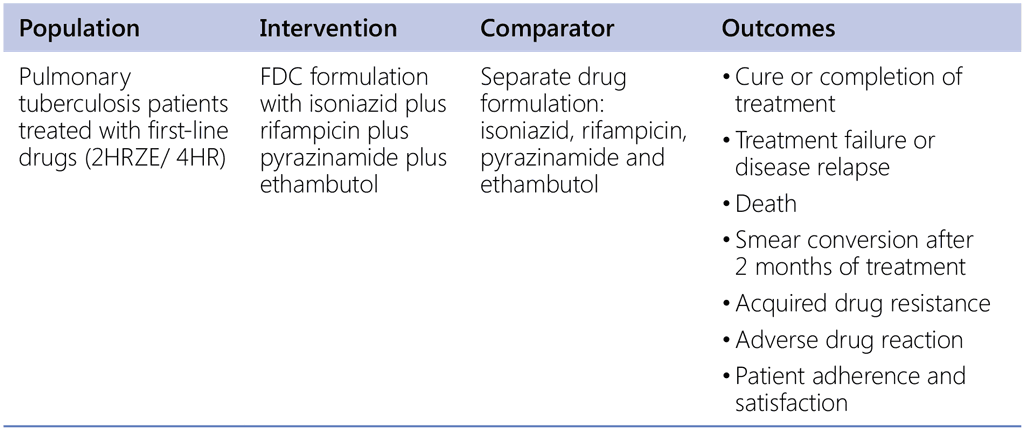
Recommendation 10. PICO question
Does the use of adjuvant corticosteroids in tuberculous meningitis provide mortality and morbidity benefits?
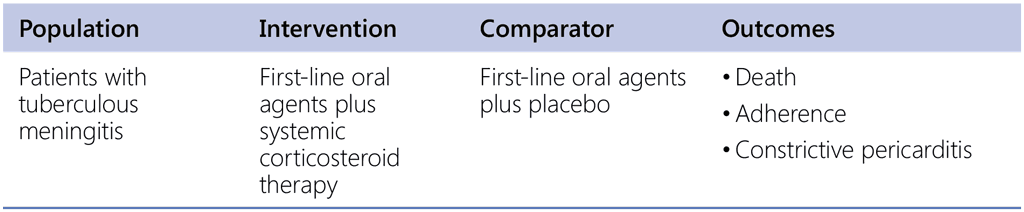
Recommendation 11. PICO question
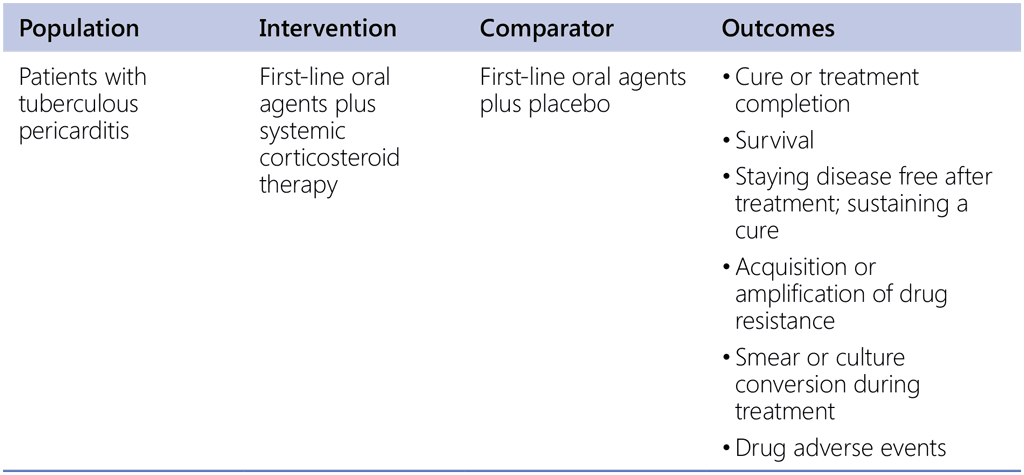
Does the use of adjuvant corticosteroids in tuberculous pericarditis provide mortality and morbidity benefits?
4.1.3. Guideline update 2022
Recommendation 7. PICO question
In patients aged ≥ 12 years with drug-susceptible pulmonary TB, is a 4-month regimen composed of rifapentine, isoniazid, pyrazinamide and moxifloxacin as effective and safe as the standard drug-susceptible TB regimen composed according to WHO guidelines?
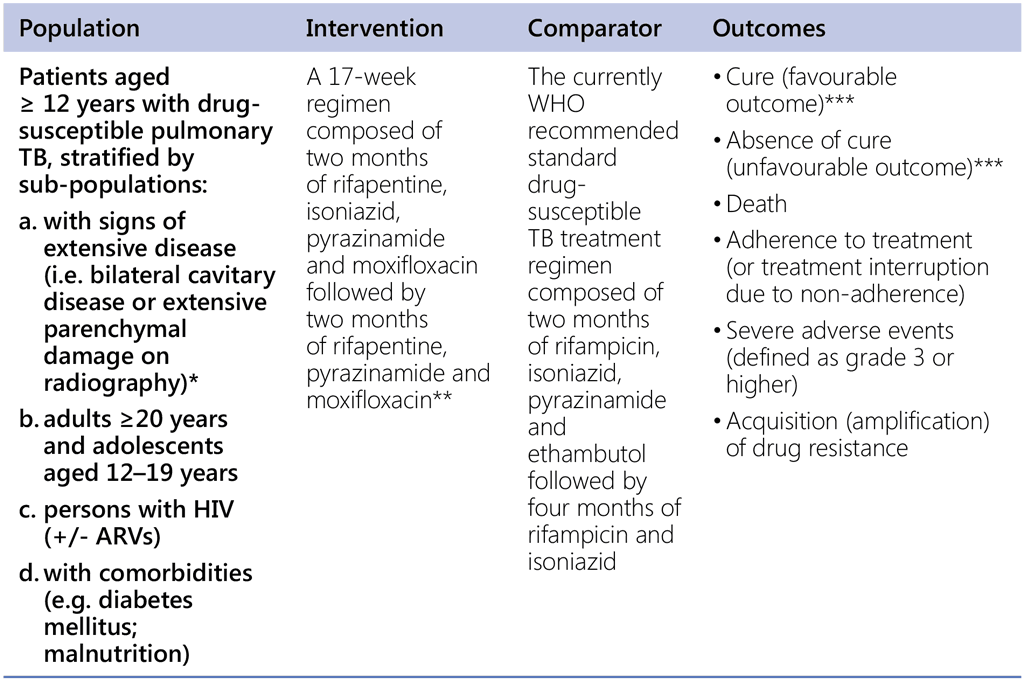
* WHO defines extensive or advanced TB disease as: presence of bilateral cavitary disease or extensive parenchymal damage on chest radiography. In children aged under 15 years, advanced disease is usually defined by the presence of cavities or bilateral disease on chest radiography.
** Standard doses (i.e. those that are currently recommended, and weight based, where relevant) of pyrazinamide, isoniazid and moxifloxacin were used and the dose of rifapentine used was 1200mg.
*** In Study 31, a participant was classified as having a favorable outcome if any one of the following conditions was met and an unfavorable outcome did not occur:
1. Participants whose last culture result during the Month 12 analysis visit window was M. tuberculosis negative.
2. Participants who were seen during the Month 12 analysis visit window and were clinically without symptoms/signs of ongoing active TB (indicated by absence of initiation of possible poor treatment response (PPTR) evaluation or PPTR that did not indicate presence of symptoms/signs of ongoing active TB), and had achieved culture conversion prior to Month 12, and
a) Were unable to produce a sputum specimen at any point during the Month 12 analysis visit window; or
b) Produced a sputum specimen that was contaminated or unevaluable without evidence of M. tuberculosis, and no sputum specimens yielded positive or negative culture results during the Month 12 analysis visit window.
A participant was classified as having an unfavorable outcome if any one of the following conditions is met:
1. A participant was considered to have absence of bacteriological cure if he/she had a sputum sample, obtained at or after Week 17 and no later than the end of the Month 12 analysis visit window, that is M. tuberculosis Culture Positive that was indistinguishable from the initial isolate (see separate sequencing plan for definitions), and this was confirmed by a second sample that was M. tuberculosis culture positive. A second confirmatory sample, on a different day without an intervening M. tuberculosis negative culture result, was required, as a single positive sputum culture result in isolation was not considered absence of bacteriological cure. If results from strain analysis were inconclusive or unavailable, it was assumed that strains were indistinguishable.
2. Participants who died from any cause during study treatment (‘study treatment phase’ is defined in the protocol), except from violent or accidental cause (e.g. road traffic accident). Suicide during study treatment was classified as an unfavorable outcome.
3. Participants who were withdrawn from follow-up or lost to follow-up prior to the scheduled end of treatment of study treatment, except for pregnancies and violent or accidental death that were instead classified as having a Not Assessable outcome (see protocol for definition).
4. Participants who had an M. tuberculosis positive culture result when last seen during or prior to the Month 12 analysis visit window, whether confirmed by a second sample or not, unless determined to have been re-infected.
5. Participants receiving any one or more of the following, except when given for failure or recurrence subsequently shown to be a reinfection with a strain of M. tuberculosis, different from that or those identified at study entry through genotyping methods):
a) Extension of treatment beyond that permitted by the protocol; excepting
a. temporary drug re-challenge;
b. over-treatment with drugs from assigned study kits;
c. twenty-one days or fewer of non-study anti-TB medications given for treatment of active TB; or
d. secondary isoniazid preventative therapy in HIV infected participants.
b) Re-start of treatment for active TB;
c) Change in treatment (including frequency or dosage) for any reason except re-infection, pregnancy, or temporary drug challenge.
6. Participants who died during the follow-up phase (as defined in the protocol) where the cause of death was considered related to TB.
Recommendation 8. PICO question
In children and adolescents with non-severe TB*, should a 4-month intervention regimen versus the standard 6-month regimen conforming to WHO guidelines be used?
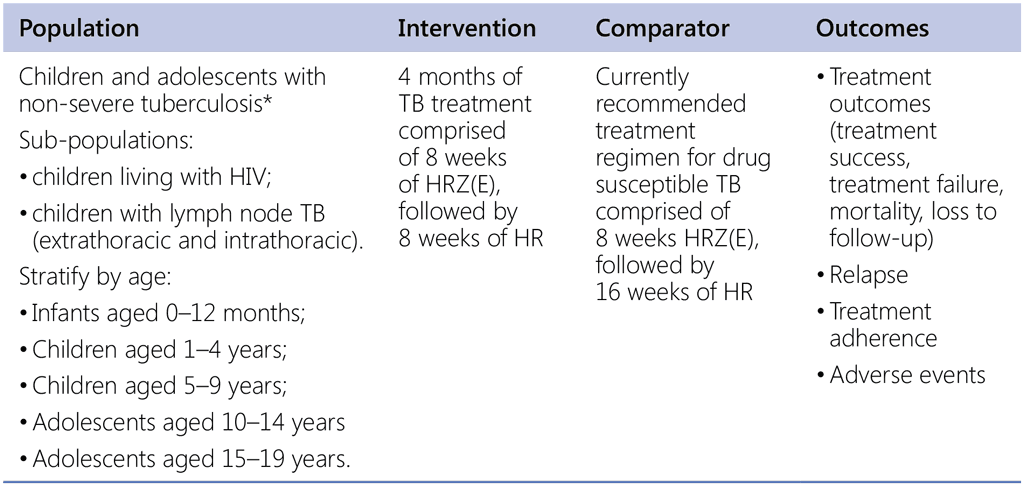
* Notes: children in whom the diagnosis of non-severe TB was established by a committee.
Non-severe TB is defined as sputum smear-negative TB, extrathoracic lymph node TB, intrathoracic lymph node TB with no significant airway obstruction, or uncomplicated forms of pulmonary TB, confined to one lobe and with no cavities.
4.2. Drug-resistant TB
4.2.1. Guideline update 2022
WHO PICO questions and comparisons
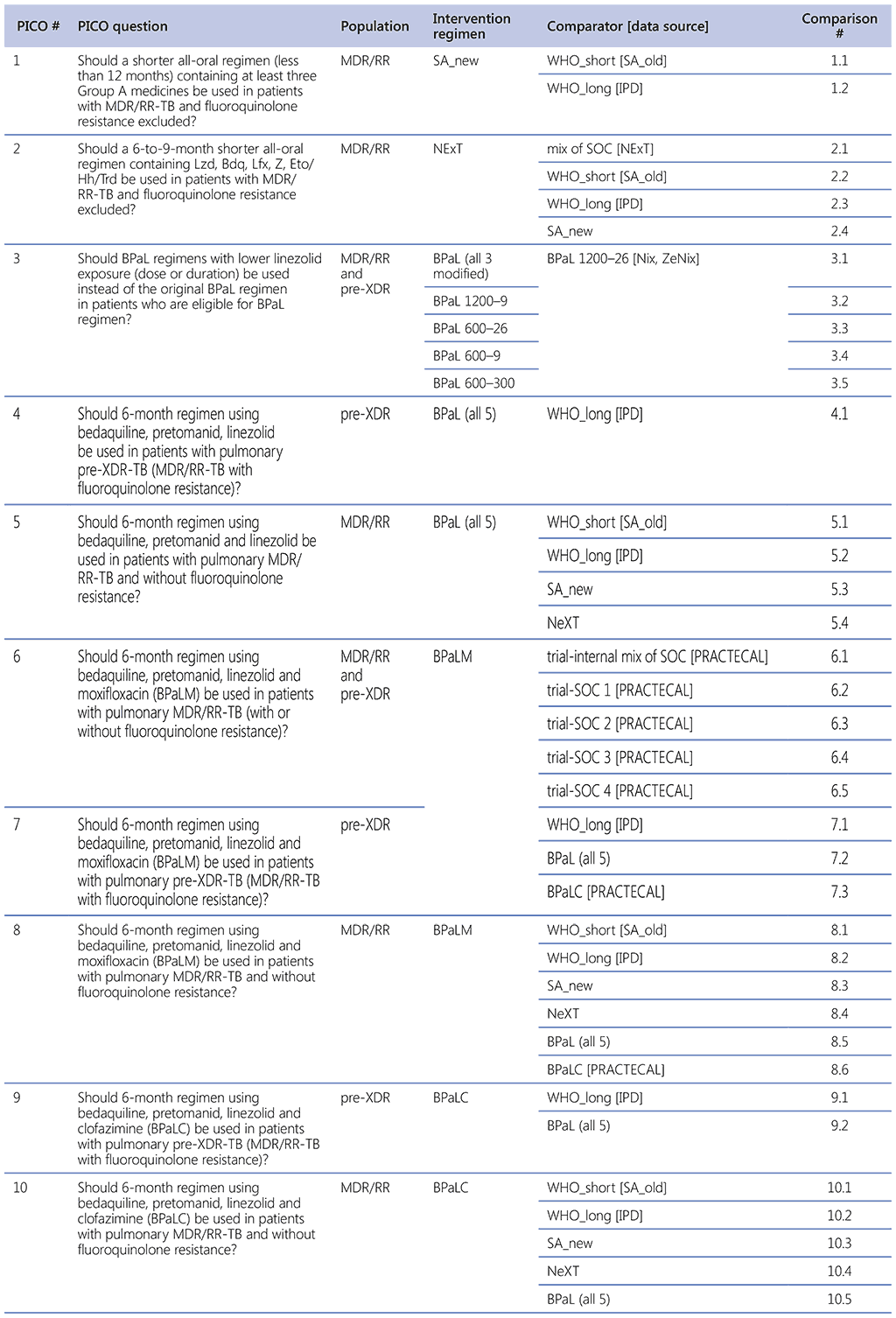
Question 1 (SA_new regimen)
Should a shorter all-oral regimen (less than 12 months) containing at least three Group A medicines be used in patients with MDR/RR-TB (fluoroquinolone resistance excluded)?
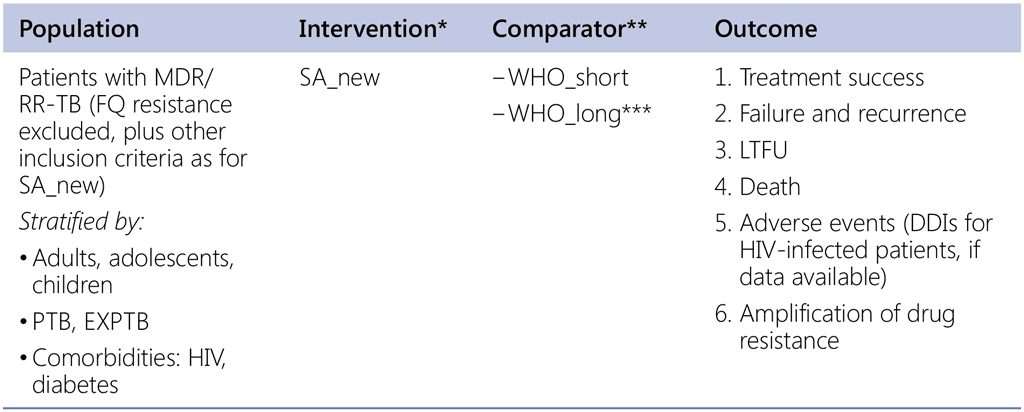
* Data sources: South Africa + OR in other countries (public call)
** Data sources:
- Shorter regimen: SA (provided by country & 2 comparator regimens in TB-PRATECAL) & other countries (public call or OR studies)
- Longer regimens: Belarus, Republic of Moldova, Georgia, Russia Federation, and others (public call)
*** If eligibility criteria for the SA_new is aligned and restricted to the same criteria as WHO_short then comparisons with the WHO_long becomes less relevant but if sufficient data we do this comparison (we may not have sufficient numbers of records with truly WHOlong, restricted to the same population as SA_new)
Note: public call for additional data is required for both intervention and comparator
Question 2 (NeXT study regimen)
- Should a 6-to-9-month shorter all-oral regimen containing Lzd, Bdq, Lfx, Z, Eto/Hh/Trd be used in patients with MDR/RR-TB (fluoroquinolone resistance excluded)?
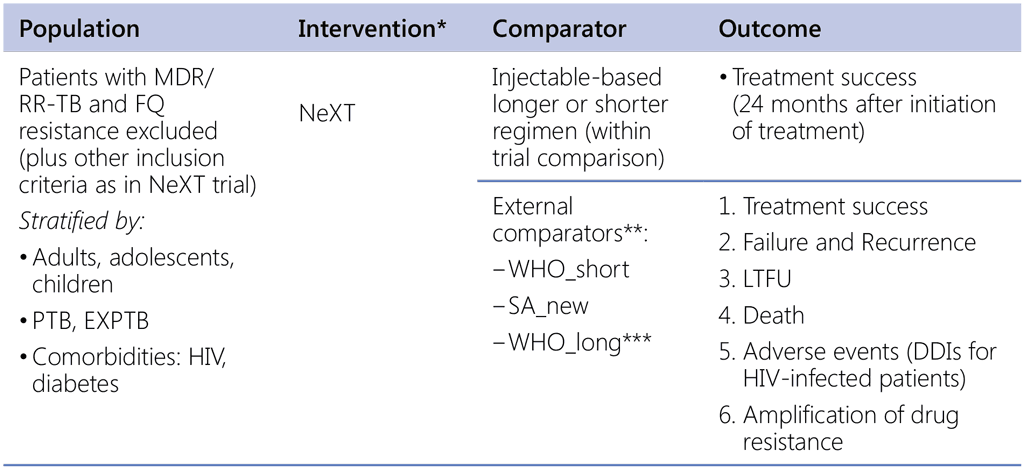
* Source of data: NExT Clinical TRial – SA. Six to nine months of all-oral regimen: Linezolid 600mg daily (reduce to 300mg if toxicity occurs), Bedaquiline 400mg for 2 weeks, followed by 200mg three times per week, Levofloxacin 750mg (<50kg) or 1 000mg (>50kg) daily, PZA 1 000–1 750mg (40–50kg) or 1 750–2 000mg (51–70kg) or 2 000–2 500mg (71–90kg) daily, Ethionamide 15mg/kg (max 900mg) daily, or high-dose Isoniazid 500mg (40–50kg) or 750mg (51–70kg) or 750–1 000mg (71–90kg) daily, or Terizidone 750mg (40–70kg) or 750–1 000mg (71–90kg) daily. Trd is used only for patients with KatG mutation (Eto and Hh no more effective)
** Data sources:
• Shorter regimens: SA (provided by NTP) & other countries (public call or OR studies)
• Longer regimens: Belarus, Republic of Moldova, Georgia, Russia Federation, and others (public call, if available)
*** If eligibility criteria for the NeXT is aligned and restricted to the same criteria as WHO_short then comparisons with the WHO_long becomes less relevant but if sufficient data we do this comparison (we may not have sufficient numbers of records with truly WHOlong, restricted to the same population as NeXT)
Background Question 1 (ZeNix and TB-PRACTECAL study regimens)
What is the safety profile of BPaL regimens with different levels of exposure to linezolid when used in MDR-TB patients?
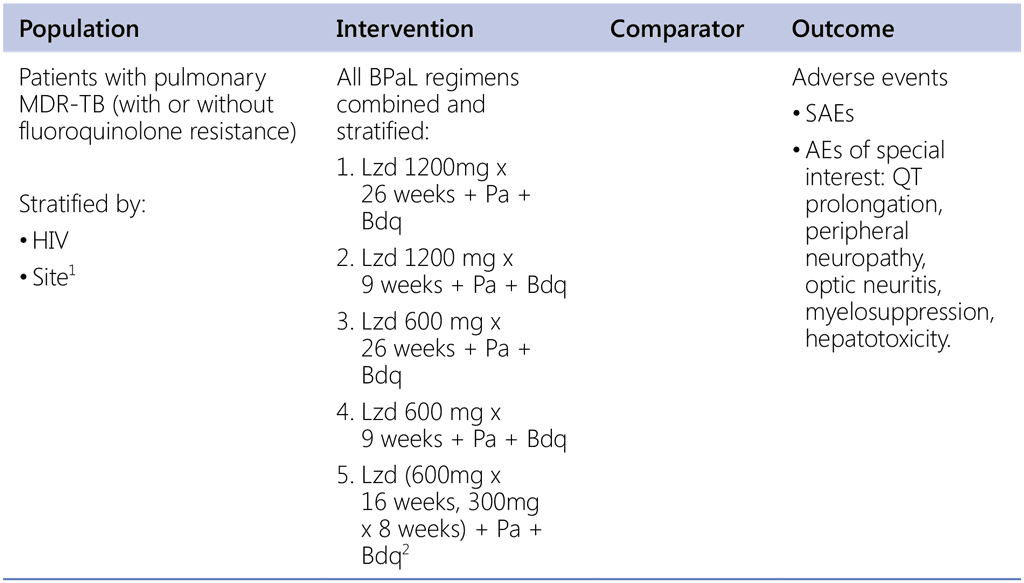
1 There is significant difference in AEs between sites in ZeNix study.
2 TB-PRACTECAL arm 3 regimen.
Question 3 (The BPaL regimens with lower Lzd exposure – ZeNix and TB-PRACTECAL regimens)
Should BPaL regimens with lower linezolid exposure (dose or duration) be used instead of the original BPaL regimen in patients who are eligible for BPaL regimen?
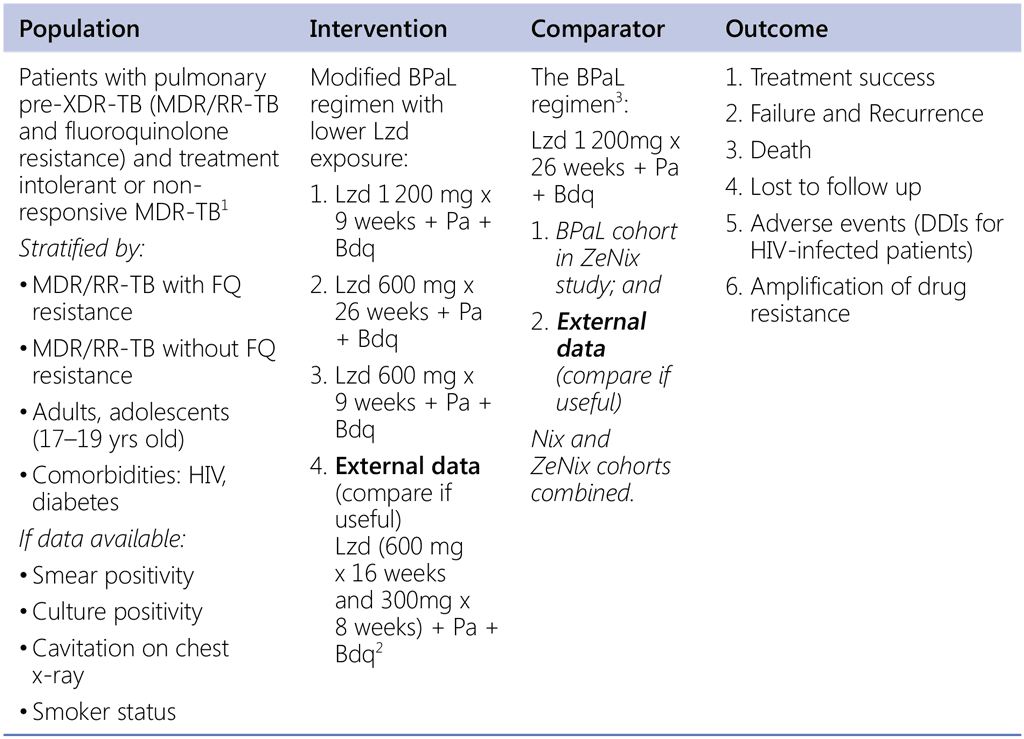
1 ZeNix study population also includes MDR/RR-TB patients with additional resistance to fluoroquinolone or injectable agents. Patients with treatment intolerant or non-responsive MDR-TB in the study are mainly intolerant or nonresponsive to the previously WHO recommended regimens (prior to the 2 020 recommendation).
2 TB-PRACTECAL arm 3 regimen – external comparator, non-randomized.
3 Data source: 1) BPaL cohort in ZeNix study; and 2) Nix and ZeNix cohorts combined. There is a difference in Bdq dosing between NiX and ZeNix studies.
Question 4. Should 6-month regimen using bedaquiline, pretomanid, linezolid be used in patients with pulmonary preXDR-TB (MDR/RR-TB with fluoroquinolone resistance)?
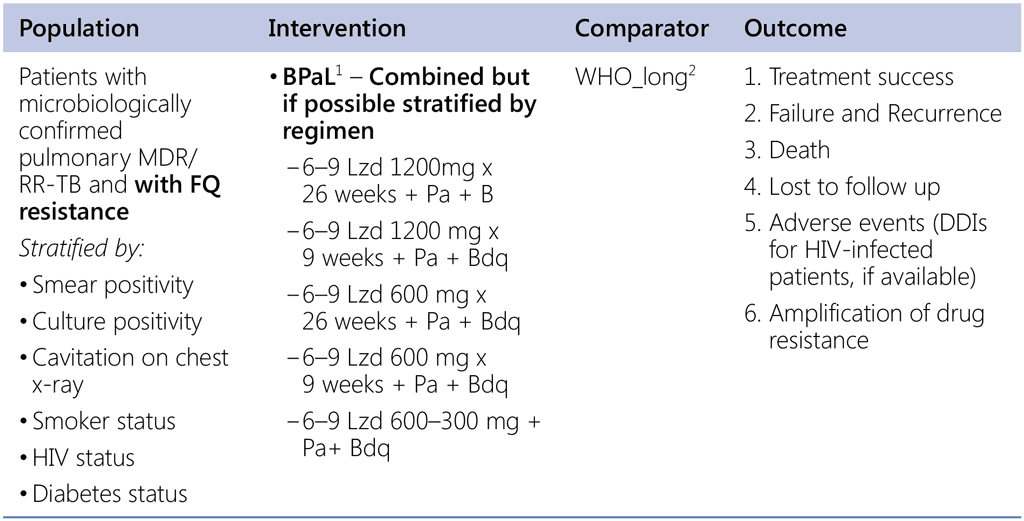
1 Nix & Zenix & PRACTECAL studies
2 Data sources:
- Data contributed by countries (public call – for LR);
- EndTB regimens (IPD);
Question 5. Should 6-month regimen using bedaquiline, pretomanid and linezolid be used in patients with pulmonary MDR/RR-TB and without fluoroquinolone resistance?
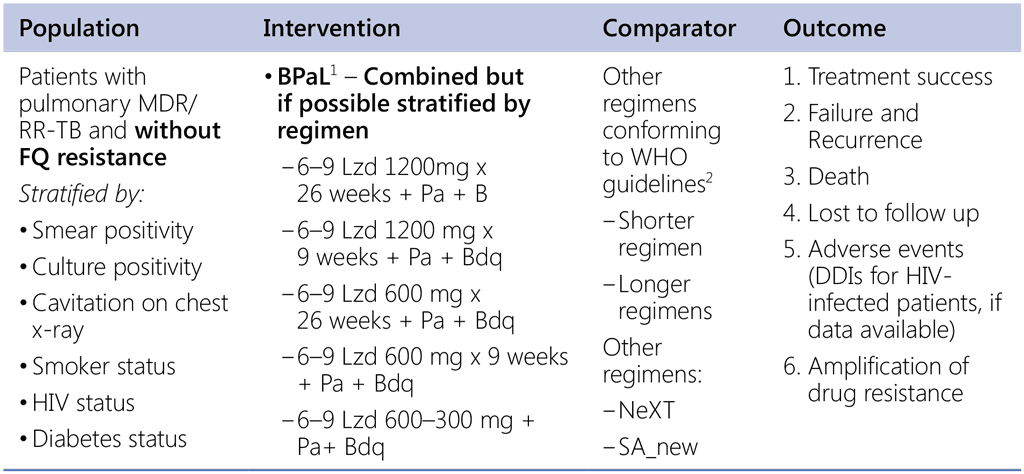
1 Nix & Zenix & PRACTECAL studies
2 Data sources:
- Data contributed by countries (public call – for both SR & LR);
- IPD: all-oral SR (from SA), EndTB regimens;
Internal TB-PRACTECAL trial comparison
Question 6. Should 6-month regimen using bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) be used in patients with pulmonary MDR/RR-TB (with or without fluoroquinolone resistance)?
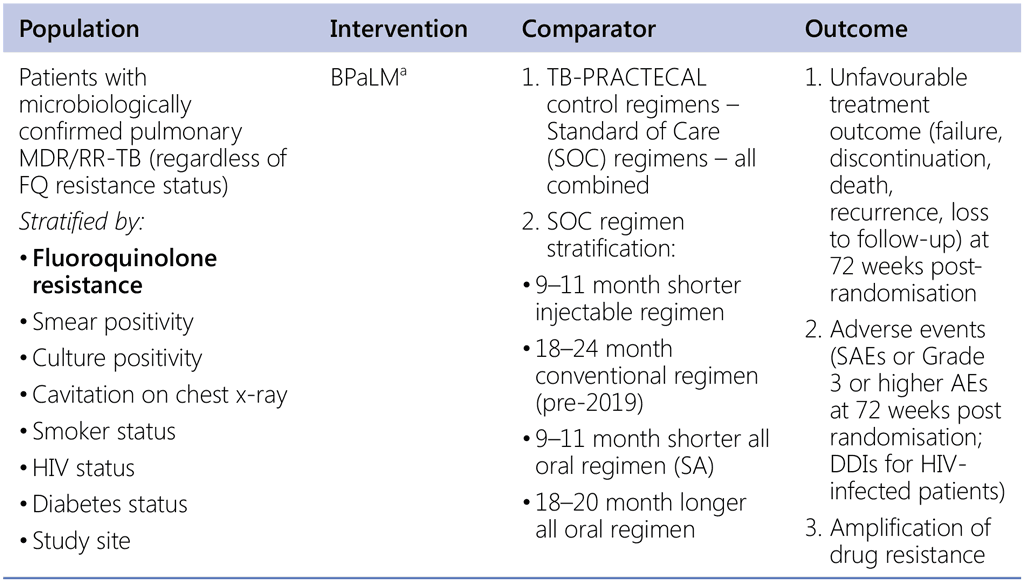
a TB-Practecal intervention arm 1: Bedaquiline: 400 mg once daily for 2 weeks followed by 200 mg 3 times per week for 22 weeks, Pretomanid: 200mg once daily for 24 weeks, Moxifloxacin: 400 mg once daily for 24 weeks, Linezolid: 600mg daily for 16 weeks then 300mg daily for the remaining 8 weeks or earlier when moderately tolerated.
TB-PRACTECAL intervention regimens vs external comparators
Question 7. Should 6-month regimen using bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) be used in patients with pulmonary pre-XDR-TB (MDR/RR-TB with fluoroquinolone resistance)?
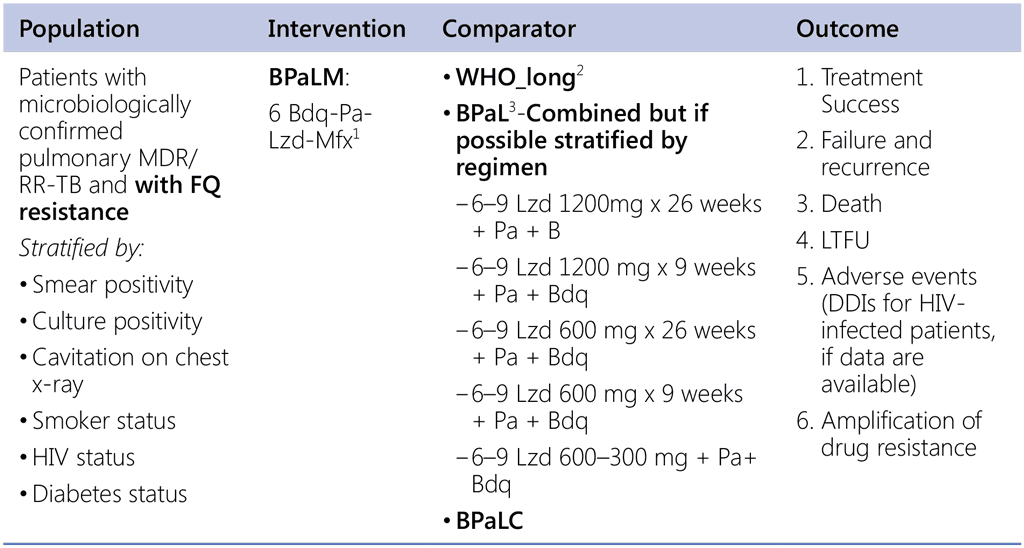
1 TB-Practecal intervention arm 1: Bedaquiline: 400 mg once daily for 2 weeks followed by 200 mg 3 times per week for 22 weeks Pretomanid: 200mg once daily for 24 weeks Moxifloxacin: 400 mg once daily for 24 weeks Linezolid: 600mg daily for 16 weeks then 300mg daily for the remaining 8 weeks or earlier when moderately tolerated.
2 Data sources:
- Data contributed by countries (public call – for LR);
- EndTB regimens (IPD);
3 Nix & Zenix & PRACTECAL studies
Question 8. Should 6-month regimen using bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) be used in patients with pulmonary MDR/RR-TB and without fluoroquinolone resistance?
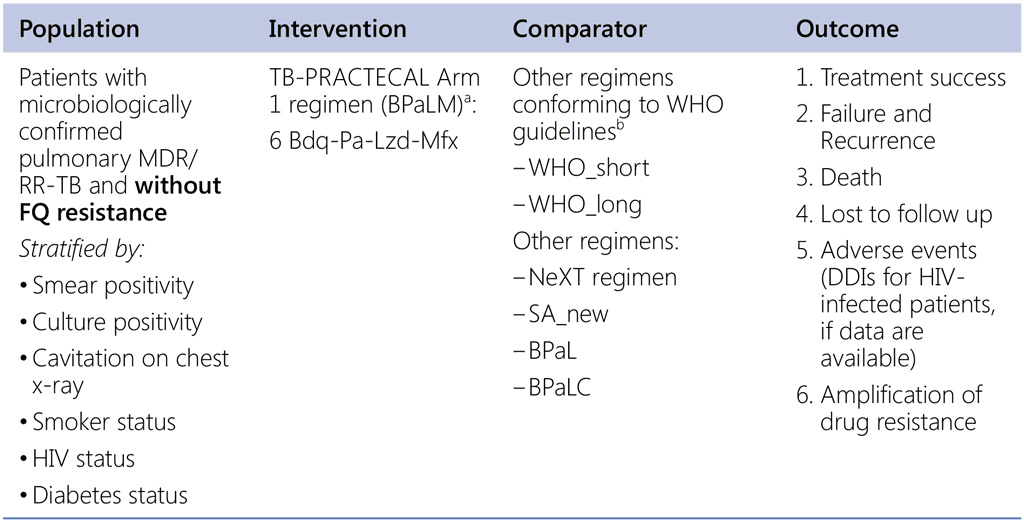
a TB-Practecal intervention arm 1: Bedaquiline: 400 mg once daily for 2 weeks followed by 200 mg 3 times per week for 22 weeks, Pretomanid: 200mg once daily for 24 weeks Moxifloxacin: 400 mg once daily for 24 weeks, Linezolid: 600mg daily for 16 weeks then 300mg daily for the remaining 8 weeks or earlier when moderately tolerated.
b Data sources:
- Data contributed by countries (public call – for both SR & LR);
- IPD: all-oral SR (from SA), EndTB regimens;
Question 9. Should 6-month regimen using bedaquiline, pretomanid, linezolid and clofazimine (BPaLC) be used in patients with pulmonary pre-XDR-TB (MDR/RR-TB with fluoroquinolone resistance)?
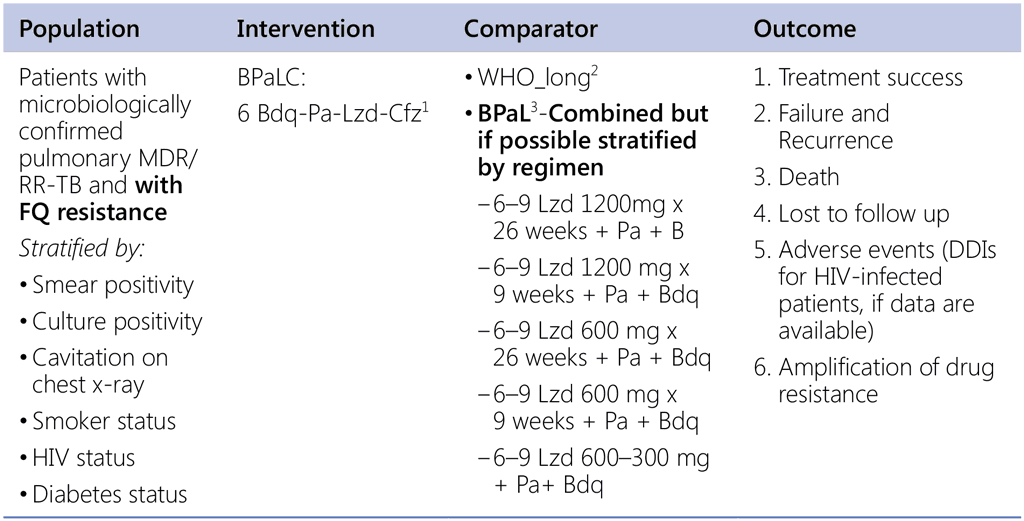
1 TB-Practecal intervention arm 2. Linezolid use: 600mg daily for 16 weeks then 300mg daily for the remaining 8 weeks or earlier when moderately tolerated.
2 Data sources:
- Data contributed by countries (public call – for LR);
- EndTB regimens (IPD);
3 Nix & Zenix & PRACTECAL studies
Question 10. Should 6-month regimen using bedaquiline, pretomanid, linezolid and clofazimine (BPaLC) be used in patients with pulmonary MDR/RR-TB and without fluoroquinolone resistance?
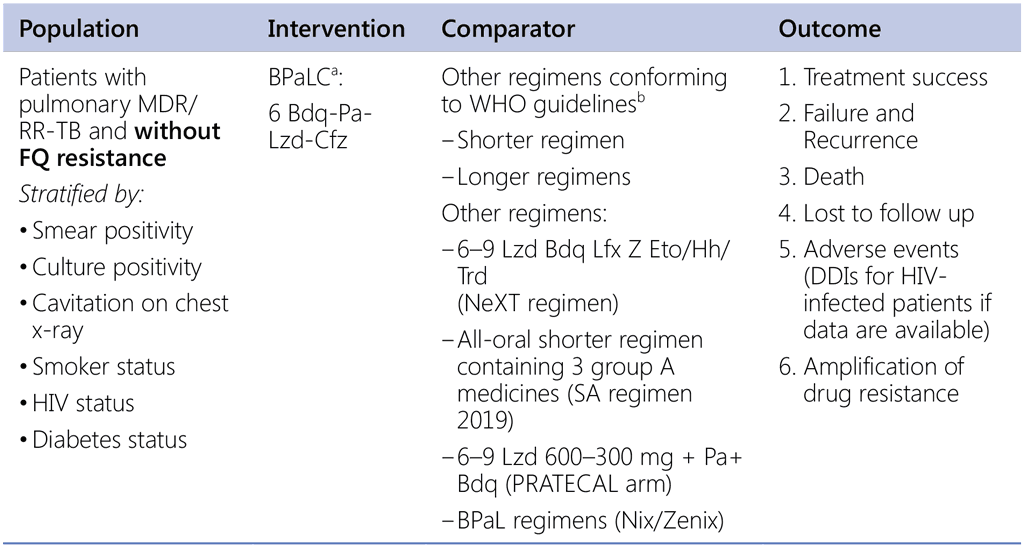
a TB-Practecal intervention arm 2, with Linezolid 600mg daily for 16 weeks then 300mg daily (or 600mg x3/wk) for the remaining 8 weeks or earlier when moderately tolerated.
b Data sources:
- Data contributed by countries (public call – for both SR & LR);
- IPD: all-oral SR (from SA), EndTB regimens;
4.2.2. Guideline update 2025
Question 1 (BEAT-TB 6-month regimen)
Should a 6-month regimen using bedaquiline, delamanid, and linezolid with or without the addition of levofloxacin or clofazimine or both (BDLLfx/C) be used in patients with pulmonary RR-TB (with or without fluoroquinolone resistance) over the currently recommended 9-month regimen?
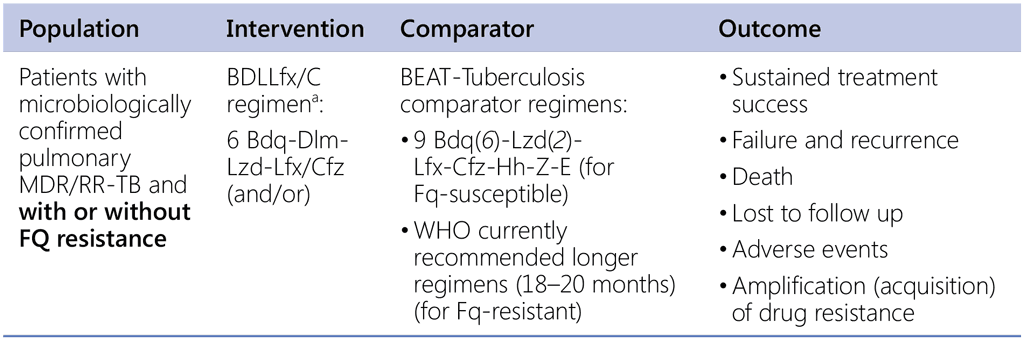
a BEAT-Tuberculosis trial intervention arm (6 months or 24 weeks)
Question 2 (endTB modified 9-month regimens)
Should any 9-month endTB trial regimens be used in patients with pulmonary RR-TB (without fluoroquinolone resistance) over the currently recommended longer regimens?
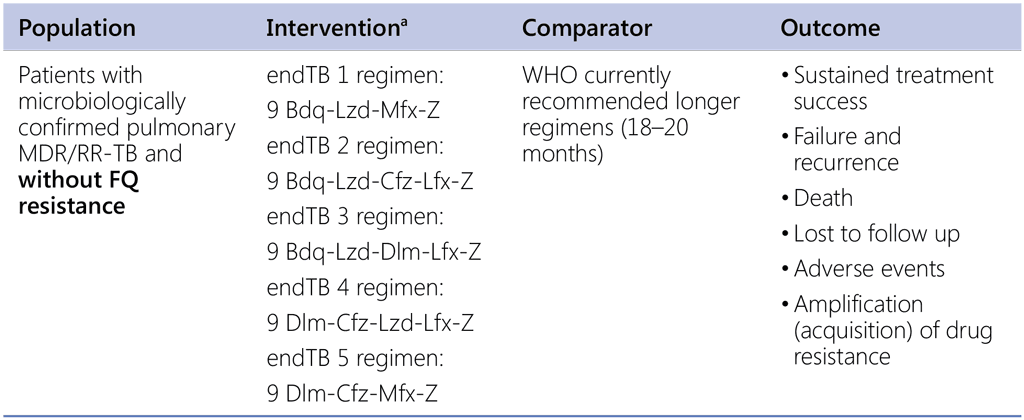
a endTB trial intervention arms (9 months or 39 weeks)
4.3. TB care and support
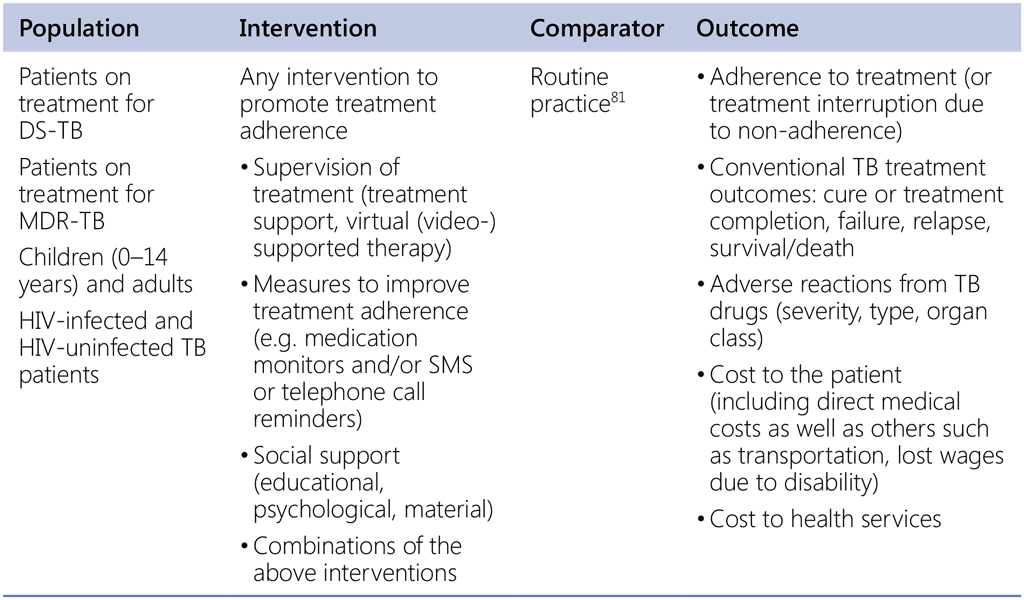
Care and support interventions for all people with TB (Guideline update 2017)
Question 1. In patients with TB, are any interventions to promote adherence to TB treatment more or less likely to lead to the outcomes listed below?
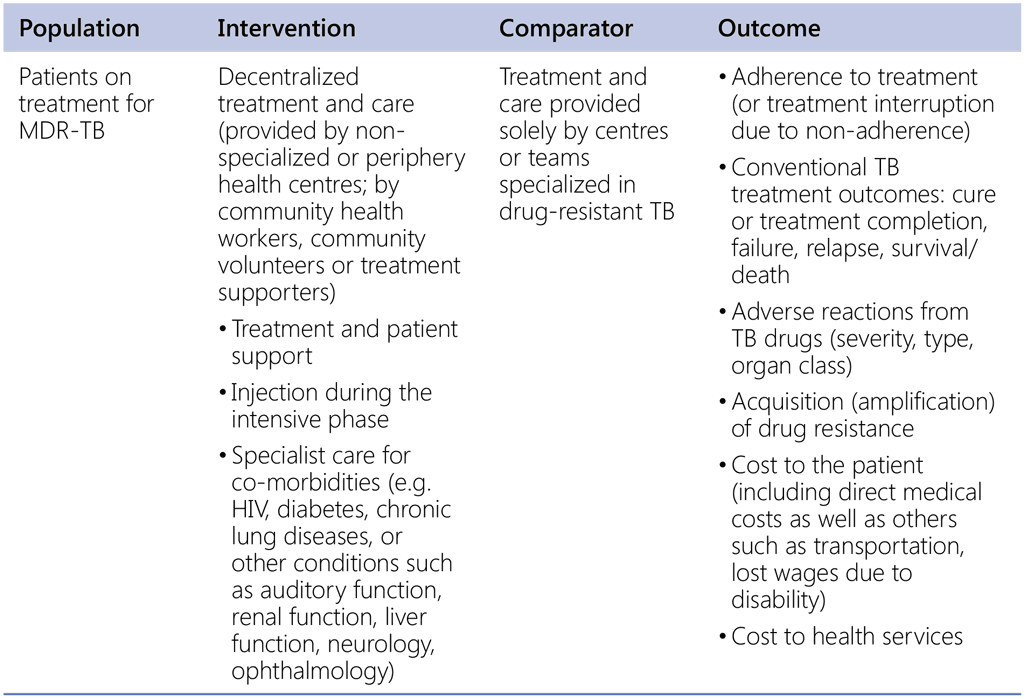
Models of care for people with drug-resistant TB (Guideline updates 2011 and 2017)
Question 2. Among patients with MDR-TB, is ambulatory therapy compared with inpatient treatment, more or less likely to lead to better outcomes?
Question 3. Is decentralized treatment and care for MDR-TB patients more or less likely to lead to the outcomes listed below?
Models of care for children and adolescents (Guideline update 2022)
Question 4. Models of care for TB case detection and TB prevention settings with a prevalence of TB in the general population of 100 per 100 000 or more:
- In children and adolescents with signs and symptoms of TB, should the decentralization of child and adolescent TB services versus centralized child and adolescent TB services (at referral or tertiary hospital level) be used?
- In children and adolescents exposed to TB, should the decentralization of child and adolescent TB prevention and care services versus centralized prevention and care services (at referral or tertiary hospital level) be used to increase coverage of TB preventive treatment in eligible children and adolescents?
- In children and adolescents with signs and symptoms of TB, should family-centred, integrated services versus standard, non-family-centred, non-integrated services be used?
- In children and adolescents exposed to TB, should family-centred, integrated services versus standard, non-family-centred, non-integrated services be used to increase coverage of TB preventive treatment in eligible children and adolescents?
81 Routine practice: regular TB drugs pick-up and consultations with physician or other health-care workers are available when necessary; TB treatment is free of charge; essential information/health education in relation to TB treatment is provided.

 Обратная связь
Обратная связь