Перекрёстные ссылки книги для 1266
All patients with DS-TB without documented resistance to isoniazid and rifampicin may be treated using the 6-month rifampicin-containing regimen 2HRZ(E)/4HR, which comprises isoniazid, rifampicin, pyrazinamide and ethambutol, for 2 months followed by isoniazid and rifampicin for 4 months (1).
This regimen is based on the historical TB treatment studies conducted by the Medical Research Council of the United Kingdom of Great Britain and Northern Ireland (United Kingdom) in the 1980s (20), and it has been widely adopted worldwide. The regimen ensures a successful outcome in about 85% of patients globally, and has a low proportion of unfavourable outcomes and adverse events (AEs), although the success rate varies among WHO regions and is lower in PLHIV (1, 4). This regimen is estimated to have averted 66 million deaths during the 20 years from 2000 to 2020.
The core WHO recommendations for the treatment of DS-TB using 6-month regimen are summarized below, with remarks.

Remarks:
A: This recommendation also applies to extrapulmonary TB except TB of the central nervous system, bone or joint for which some expert groups suggest longer therapy.
B: WHO recommends that national TB control programmes provide supervision and support for all TB patients to ensure completion of the full course of therapy.
C: WHO recommends drug-resistance surveys (or surveillance) for monitoring the effectiveness of the treatment programme, and for designing standard regimens.
WHO recommends daily dosing as the best frequency throughout the entire course of treatment, as included in the recommendation below:

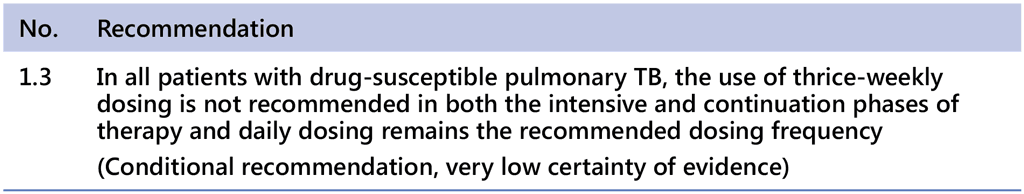
Daily administration reduces the rate of acquired drug resistance by up to 3.3 times when comparing patients who received a daily regimen for the entire duration with those who received intermittent dosage (1). The effect of a patient missing one or more doses (either accidentally or due to stock-outs) is much more significant if the regimen is intermittent. The term “daily” indicates an intake of anti-TB drugs for 7 days per week. In patients with DS-TB, WHO does not recommend thrice-weekly dosing for either the intensive or the continuation phase of treatment as described in this recommendation:
WHO recommends the use of FDC tablets, as included in the recommendation below:

WHO recommends not prolonging the continuation phase of treatment of the 6-month regimen in new pulmonary TB patients if a sputum smear is found to be positive at the end of the intensive phase of treatment, as included in the recommendation below:

3.1 Eligibility
Any patient – whether a child or an adult – with DS-TB is eligible for this regimen. The regimen is considered safe for pregnant women; it can also be used in children of all ages, although ethambutol can be omitted for patients who are HIV-negative or in settings with a low prevalence of HIV or isoniazid resistance. Patients without a history of TB disease and treatment are less likely to have strains resistant to first-line medicines, although infection by the resistant strains often cannot be ruled out, especially in resource-limited settings. Where possible, it is best to ascertain susceptibility to the medicines used; susceptibility to isoniazid and rifampicin (the most potent drugs in the regimen) is particularly important.
In patients with evidence of resistance to isoniazid or rifampicin, this regimen cannot be used; instead, a specific regimen needs to be designed, as described elsewhere (17).
3.2 Composition and duration of the regimen 2HRZE/4HR
The WHO guidelines recommend treating people with DS-TB with a 6-month regimen composed of four first-line TB medicines: isoniazid, rifampicin, pyrazinamide and ethambutol (1). The regimen is a combination of those four drugs (i.e. HRZE) for 2 months followed by isoniazid and rifampicin (i.e. HR) for 4 months, administered daily. In children (usually defined as being aged <10 years) in settings with a high background prevalence of isoniazid resistance or HIV infection, or in CLHIV, ethambutol should be used in the first 2 months of treatment; in all other situations ethambutol can be omitted, resulting in a 2HRZ/4HR regimen (19).
As a general rule, WHO does not recommend prolonging the regimen beyond 6 months (1), because there is evidence that prolongation does not significantly increase efficacy. The first 2 months of treatment, which includes four drugs, is usually enough for the strong bactericidal activity of this regimen to be effective. Thus, the presence of one or more sputum smear results that are still positive after 2 months usually indicates the presence of dead bacilli; however, in some cases, it might be due to undetected resistance to one or more drugs. If the patient is not improving clinically and radiologically, and drug resistance or potential failure is suspected, rapid diagnostic testing to exclude these scenarios should be undertaken promptly, together with culture and DST, to provide a basis for any adjustment of the treatment strategy (21).
The systematic reviews on the dosages of the first-line medicines (rifampicin, isoniazid, ethambutol, and pyrazinamide) used in the treatment of drug-susceptible tuberculosis in adults and children were conducted. The reviews concluded that the WHO-recommended doses for rifampicin, isoniazid, ethambutol and pyrazinamide remain valid in adults and children. (Annexes 1 and 2)
3.3 Considerations for implementation
The 6-month rifampicin-based regimen is the standard regimen for the treatment of DS-TB in many countries and has been for many years; thus, there is a great deal of experience in using this regimen.
Rapid diagnostic testing and universal DST is a recommended target for all NTPs (3). In settings where DST results are not yet routinely available to guide the management of individual patients, patient history and clinical judgement are used to make decisions on the empirical use of this regimen.
Diagnostic challenges include being a long distance from the facilities where quality TB diagnostics are available, technical difficulties in implementing these tests and difficultly in accessing health services. The coronavirus disease (COVID-19) pandemic has further complicated rapid and universal access to quality TB diagnosis. Also, diagnosis of TB in children is particularly challenging.
NTPs should obtain and use their country-specific drug-resistance surveillance (DRS) to estimate the level of MDR/RR-TB. Periodic drug-resistance surveys or ongoing surveillance in each country are essential for monitoring the impact of the regimen and the overall treatment programme (1).
To improve treatment adherence and minimize the acquisition of MDR/RR-TB, it is critical for NTPs to ensure adequate treatment support in the context of patient-centred care. Implementing treatment support and care requires resources to ensure optimal adherence and provide patient education and counselling (1). It is important to educate and support patients, to ensure that they complete treatment with all the prescribed doses within the planned period of time (1).
Daily dosing is considered optimal because it reduces the probability of selecting resistant mutants. However, such dosing may be challenging for NTPs in terms of providing daily treatment support.
The use of FDCs may provide programmatic benefits by making it easier to order medicines, simplifying supply chain management, reducing the occurrence of stock-outs, and facilitating drug delivery and prescription. FDCs with proven bioavailability may also provide additional benefits, especially in settings with many TB patients and a limited number of health care workers, because FDCs reduce the need for additional health care staff and for training in dosing and dispensing of medications, while contributing to a lower pill burden for patients. However, because use of FDCs lacks the flexibility that is available when using loose tablet formulations, FDCs do not always provide optimal dosing in all individuals (1).
NTPs need to procure a quantity of loose or single drug formulations for certain treatment conditions. Having single drug formulations available would also be beneficial to programmes in cases of adverse reactions to TB medications, when drugs must be reintroduced one at a time (see Section 8 for details) (1).
3.4 Subgroups
This 6-month regimen can be used in all subgroups, including PLHIV and children. This regimen can also be used in patients with extrapulmonary TB, except those with TB affecting the central nervous system or with osteoarticular forms of TB.
3.4.1 People living with HIV
The interactions of rifampicin (the mainstay of TB treatment) with antiretroviral therapy (ART) are of concern in HIV-associated TB. When the 6-month rifampicin-containing regimen is used, these drug interactions may result in decreased concentrations of antiretroviral drugs. In 2016, WHO published key considerations for managing concomitant TB and HIV therapy (22). Standard, rifampicin-containing anti-TB treatment was recommended in combination with efavirenz-based ART. Conversely, key contraindicated drug combinations were rifampicin with nevirapine and protease inhibitors. In people with HIV-associated TB receiving these drugs, rifabutin (where available) was suggested as a suitable substitute for rifampicin.
Rifampicin is known to lower plasma concentrations of the HIV medication dolutegravir. This has led to concerns about efficacy and the development of HIV resistance due to lower levels of dolutegravir. In such cases, WHO guidelines recommend adjusting the dose by offering 50 mg of dolutegravir twice per day (instead of a single daily dose of 50 mg) (22). These recommendations are still in place, although evidence that doubling the dose of dolutegravir might not be necessary is emerging. A study from Botswana demonstrated the efficacy and safety of a standard-dose dolutegravir-based regimen compatible with an efavirenz-based regimen for HIV-positive TB patients who received rifampicin (23).
3.4.2 Children
Children with suspected or confirmed pulmonary TB or tuberculous peripheral lymphadenitis who live in settings with a low prevalence of HIV or of isoniazid resistance, or children who are HIV-negative, can be treated with a three-drug regimen (HRZ) for 2 months followed by a two-drug regimen (HR) for 4 months at the dosages specified in Annex 4. Treatment may require dose adjustment to reconcile the effect of age and possible toxicity in infants (19).
The reduced pill burden afforded by using the recommended FDCs may be especially valuable in patients with comorbidities (notably HIV infection) and in paediatric patients (who may have some difficulty in swallowing a large quantity of medications). Therefore, the availability of palatable dispersible formulations specifically tailored to children is of paramount importance.
Patients with some specific medical conditions (e.g. intolerance to certain TB drugs, or impairment of liver or renal function) are likely to require individualized adjustment of medication dose; however, this can only be done with single drug formulations.
3.5 Treatment monitoring
Standard treatment monitoring should be ensured to assess the treatment response and any AEs.
The available tools for treatment monitoring are bacteriological examinations (sputum smear, culture and DST), chest radiography (CXR) and clinical examination by the treating physician.
The important timepoints of the necessary TB monitoring examinations are after 2 months of treatment (especially if the patient does not improve, and underlying drug resistance and possible failure are suspected) and at the end of treatment.
If the sputum specimen obtained at the end of the intensive phase of treatment (i.e. end of month 2) is positive on smear microscopy, and the patient does not show clinical and radiological improvement, sputum culture and DST should be performed. Based on these results, the patient should be reassessed to identify possible risk factors for failure and the treatment strategy should be changed if necessary.
Culture and DST are important for determining whether the bacilli are alive and whether any previously undetected resistance is present.
Malabsorption of drugs and drug–drug interactions (DDIs) can occur, especially in PLHIV or those with diabetes, in critical care or receiving concomitant medications. Where the clinician suspects malabsorption, it is useful to undertake evaluation and monitoring of the blood levels of the drugs composing the regimen; this can be done using therapeutic drug monitoring (24). Section 9 provides additional details on clinical monitoring in cases of AEs due to anti-TB drugs, and on treatment monitoring with sputum smear, culture and radiology.

 Обратная связь
Обратная связь