Перекрёстные ссылки книги для 1266
Three Phase III trials (i.e. REMoxTB, OFLOTUB and RIFAQUIN) failed to demonstrate non-inferiority of shorter regimens used to treat DS-TB (25–27). The recent Phase III trial Study 31 (1) assessed the safety and efficacy of two 4-month regimens for the treatment of DS-TB (28). Patients from 13 countries were recruited for this multicentre, open-label, three-arm non-inferiority RCT, which was carried out in adolescents and adults (aged ≥12 years) with smear and culture positive pulmonary DS-TB (28).
The 4-month rifapentine-moxifloxacin arm demonstrated non-inferiority when compared with the standard of care (the WHO-recommended 2HRZE/4HR regimen). The primary efficacy end-point of Study 31 was TB disease-free survival at 12 months after randomization, whereas the primary safety end-point was the proportion of participants with Grade 3 or higher AEs during the study’s drug treatment.
The proportion of patients who were cured was 84.5%, with 99.7% retention on treatment and 0.4% all-cause mortality recorded within 14 days of the end of treatment. Grade 3 or higher AEs were noted in 18.8% of participants in the rifapentine-moxifloxacin arm compared with 19.3% in the standard 2HRZE/4HR regimen (28).
The slight difference in all-cause mortality and AEs during treatment, and the slight increase in retention on treatment compared with the 6-month 2HRZE/4HR regimen allowed WHO to recommend this shorter regimen, as follows:
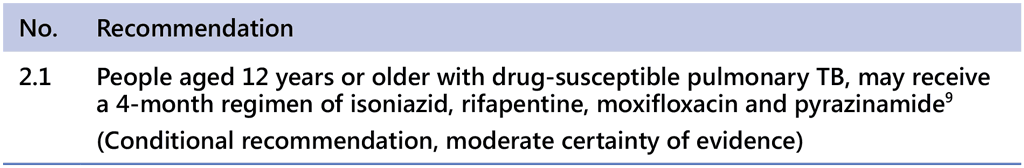
4.1 Eligibility
Adults and children aged 12 years or older with a body weight of more than 40 kg and affected by pulmonary DS-TB are eligible for this regimen, including those who are also HIV-positive with a CD4 count of more than 100 cells/mm3 and patients with diabetes. The following exceptions, detailed in Section 4.4.2, should be highlighted:
- patients weighing less than 40 kg;
- patients with severe extrapulmonary TB (e.g. tuberculous meningitis, disseminated TB, osteoarticular TB or abdominal TB);
- PLHIV with a CD4 count of less than 100 cells/mm3;
- children and adolescents aged under 12 years; and
- pregnant, breastfeeding and postpartum women.
4.2 Composition and duration of the regimen
The regimen evaluated by Study 31 comprised 8 weeks of daily isoniazid, rifapentine, moxifloxacin and pyrazinamide, followed by 9 weeks of daily isoniazid, rifapentine and moxifloxacin (2HPMZ/2HPM).
For this regimen, daily dosing (i.e. 7 days per week, as used in Study 31) is suggested, taking advantage of a treatment supporter or video-supported treatment (VST).
The dose of rifapentine used was fixed at 1200 mg and moxifloxacin at 400 mg. Other medicines were provided at standard recommended doses (Annex 4). The study was based on the regimen with moxifloxacin; therefore, replacement of moxifloxacin by another FQ cannot be recommended.
As is the case for other regimens, WHO does not recommend prolonging the regimen beyond the planned duration of 4 months.
The first 2 months of treatment, which includes four drugs, is usually enough for the strong bactericidal activity of this regimen to be effective. Thus, the presence of one or more sputum smear results that are still positive after 2 months usually indicates the presence of dead bacilli; however, in some cases, it might be due to undetected resistance to one or more drugs. If the patient is not improving clinically and radiologically, and drug resistance or potential failure is suspected, rapid diagnostic testing to exclude this possibility should be undertaken promptly, together with culture and DST, to provide a basis for any adjustment of the treatment strategy.
4.3 Considerations for implementation
Several factors need to be considered when deciding on the implementation of this regimen: DST, treatment support, pill burden, cost of medicines, administration of the shorter regimen with food, training of health care workers and criteria guiding the choice of regimen. These factors are discussed below.
DST
Although DST use must, in principle, be universal, it is not yet available in all settings. However, rapid DST for key medicines, including isoniazid, rifampicin and the FQ, is rapidly expanding. WHO recommends rapid genotypic testing for TB and RR-TB as an initial test at diagnosis; if DST for the FQ and isoniazid can be performed at the same time, this can make it easier to allocate the most appropriate regimen, although the testing has implications for costs, logistics and laboratory workload.
In practical terms, although highly desirable, baseline DST for FQ would not be necessary for patients with confirmed rifampicin-susceptible TB by a reliable, WHO-recommended rapid molecular diagnostic test. The prevalence of FQ resistance in patients without confirmed rifampicin resistance is usually low (5–9) but can reach 15% in patients with documented DR-TB (4). In settings where the prevalence of resistance to FQ in patients with DS-TB is higher because of their widespread use for other conditions, DST for the FQ would be highly recommended at baseline to exclude FQ resistance.
Treatment support
In Study 31, patients received treatment 7 days per week. At the early stages of the introduction of this regimen, treatment support with observation may be important given the current pill burden and the lack of an FDC formulation. Current WHO recommendations support the use of observation but also other forms of patient support; overall, even though this regimen is shorter, patient support remains a key element of TB programming.
Pill burden
Currently, the overall pill burden will be high for patients who receive this 4-month regimen, because there is no FDC tablet for this regimen. This may affect acceptability by patients; however, this situation may change as uptake of this regimen improves, creating a demand for the regimen and its component medicines.
Cost of medicines
Currently, the cost of the shorter regimen is substantially higher than that of the 6-month 2HRZE/4HR regimen, mainly due to the inclusion of rifapentine. Again, this situation may change as uptake of this regimen improves, creating a demand for the regimen and its component medicines. Several pharmaceutical companies are ready to bring quality-assured rifapentine to the market, including generic forms. The availability of rifapentine for this regimen may also depend on the uptake of rifapentine-containing regimens for TB prevention.
Administration of the shorter regimen with food
In some settings, administration of the shorter regimen with food may present a challenge. In Study 31, a flat dose of 1200 mg of rifapentine was given daily, with food. Pharmacokinetic and pharmacodynamic modelling predicted that a rifapentine dose of 1200 mg without food would yield an area under the curve (AUC) similar to that of a rifapentine dose of 900 mg with a high fat meal. Given that the target rifapentine AUC lies somewhere between that achieved with a very high fat meal and a rifapentine dose of 900–1200 mg, the strategy proposed was a rifapentine dose of 1200 mg with a modest food requirement, the rationale that a very high fat meal may not be feasible under routine TB care conditions, whereas dosing with food may be feasible. For some medicines (e.g. moxifloxacin), food may delay absorption.
Training of health care workers
Training will be necessary when introducing the 4-month regimen into a programmatic setting. However, this is a requirement for any new programmatic intervention, and the ability to shorten treatment and potentially treat more patients may offset the initial investments in training.
Criteria guiding the choice of regimen
Eligibility criteria for the regimen, age and patient preference should guide the choice between the 6-month and 4-month regimens. Other local factors can be important, such as the availability and cost of rifapentine.
4.4 Subgroups
Data from Study 31 allowed subgroup analyses for four patient groups: PLHIV, people with diabetes, people with a low body weight (i.e. a body mass index [BMI] <17.9 kg/m2) and patients with extensive pulmonary TB disease (using a cut off of >50% lung parenchyma affected) on CXR. Although no statistically significant differences appeared when comparing the 4-month regimen to the current standard 2HRZE/4HR regimen, the number of patients in some of these subgroups was small (1).
Additional pharmacokinetic analyses being undertaken by the trial investigators will be available in the future and may provide additional information on drug exposures in these groups. Other subgroup analyses conducted as part of the trial included analyses by age group, sex, presence of cavities, cavity size, sputum smear grade, smoking history, Xpert® cycle threshold values and time to positivity (days) with the mycobacterial growth indicator tube (MGIT) liquid culture automated system.
4.4.1 Subgroups in which the shorter regimen can be used
The shorter regimen can be used in PLHIV, people with diabetes (although the evidence is modest), people with extensive pulmonary TB disease, and children and adolescents (1).
People living with HIV
Study 31 included a sufficient proportion of PLHIV (about 8%), most of whom were on ART. Thus, sufficient evidence is available to support the use of the regimen when the CD4 count is not below 100 cells/mm³.
People with diabetes
There are scant data on the use of this regimen among people with diabetes, but additional information from pharmacokinetic analysis will become available in the future. Thus, although the shorter regimen may be considered as an option, it may be prudent to monitor this patient group closely for hepatotoxicity, and eventually consider therapeutic drug monitoring whenever feasible, if malabsorption is suspected (because of diabetes or interactions with hypoglycaemic drugs). More information on the regimen’s effectiveness in this group will also be important because diabetes is common in some countries. Additional information on the management of patients with liver problems is given in Section 8.
People with extensive pulmonary TB disease
The trial reported on the presence of cavitation and the extent of disease on CXR, as a percentage and cavity size (absent, <4 cm or ≥4 cm). For some subgroups, there was limited or no evidence on the use of the shorter regimen, but the use of this shorter regimen could be considered because favourable outcomes were reported using it in patients with extensive pulmonary disease.
Children and adolescents
The 4-month regimen including rifapentine and moxifloxacin (2HPMZ/2HPM) may be selected for adolescents aged 12 years and over and weighing at least 40 kg with pulmonary TB, regardless of disease severity (29). Factors to consider before selecting this regimen are that:
- the regimen should not be used in children and adolescents aged under 12 years; and
- the regimen should not be used in adolescents with forms of extrapulmonary TB such as tuberculous meningitis, disseminated (miliary) TB, osteoarticular TB or abdominal TB.
4.4.2 Subgroups in which the regimen is not recommended
For some subgroups, there was no evidence (because they were ineligible for inclusion in the trial). The use of the shorter regimen outside of the research environment is not indicated in the subgroups giving below.
Patients weighing less than 40 kg
Low body weight can indicate severe forms of TB disease; therefore, close follow-up and use of the 6-month regimen may be preferable in this subgroup, as there is more experience with this regimen.
Patients with extrapulmonary TB
In patients with extrapulmonary TB – such as tuberculous meningitis, disseminated (miliary) TB, osteoarticular TB and abdominal TB – the regimen is not recommended.
PLHIV with a CD4 count of less than 100 cells/mm³
A low CD4 count is indicative of severe immunosuppression, leading to concerns about an increased risk of relapse in this group.
Children and adolescents aged under 12 years
There is presently no evidence on the use of this regimen in children and adolescents aged under 12 years.
Pregnant, breastfeeding and postpartum women
There is currently no evidence available on the use of this regimen in women who are pregnant, breastfeeding or postpartum.
4.5 Treatment monitoring
The current guidance on monitoring the response to treatment of DS-TB is unchanged. WHO does not recommend baseline electrocardiogram (ECG) monitoring for those receiving the shorter regimen (unless clinically indicated), and laboratory monitoring such as liver function tests (LFT) is the same for both regimens (1). Some countries may have different requirements for LFT and ECG monitoring because of the “black box” warnings for moxifloxacin (related to QTc prolongation). Clinical monitoring is recommended in some countries for rare but possible AEs related to moxifloxacin that are common to other FQ (e.g. tendonitis, Clostridium difficile diarrhoea and peripheral neuropathy) and such monitoring should be carried out according to the country’s policies.
NTPs need to monitor patients’ condition with regular clinical follow-ups and may perform at least smear microscopy after 2 months of treatment to monitor treatment response bacteriologically. Lack of clinical or bacteriological response to treatment may need to trigger further clinical and radiological assessment, complemented by sputum smear culture and DST. Although the regimen can be continued while awaiting results of these assessments, once the results are available they will provide the clinician with the evidence to change the regimen or treatment strategy. Additional information on treatment monitoring is given in Section 9.
9 Two months of isoniazid, rifapentine, moxifloxacin, and pyrazinamide, followed by two months of isoniazid, rifapentine, and moxifloxacin

 Обратная связь
Обратная связь