Перекрёстные ссылки книги для 1266
Recommendations on DS-TB treatment and ART in PLHIV:
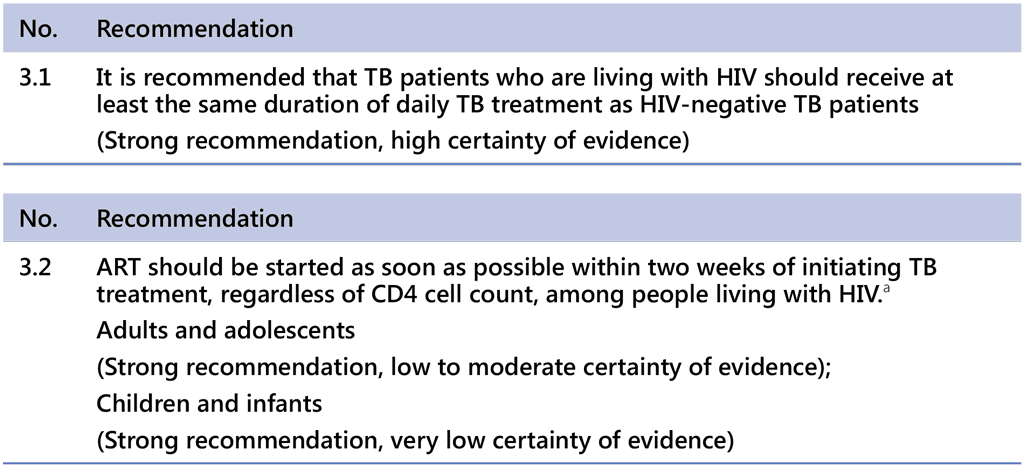
a. Except when signs and symptoms of meningitis are present.
Patients with HIV infection and TB have an increased risk of death, treatment failure and relapse (4). There is evidence that PLHIV with TB coinfection who are treated with ART respond much better to anti-TB treatment and have improved outcomes; therefore, ART is of paramount importance (22, 35).
6.1 Eligibility
The recommendation on starting ART in TB patients has recently been expanded to include all patients, regardless of CD4 count. Although all three regimens (Table 1.2.1) can be initiated in PLHIV, the 6-month regimen is a preferred option in those with a CD4 count of less than 100 cells/mm3.
6.2 Composition and duration of the regimen
All people living with HIV with DS-TB may be treated using the same duration of TB treatment as HIV-negative TB patients. There is much experience of treating these patients with the 6-month rifampicin-containing regimen 2HRZE/4HR (1, 36). The 4-month regimen with rifapentine and moxifloxacin has also been shown to perform well in patients who are also HIV-positive (1). The evidence on the use of this 4-month regimen in people living with HIV was limited to those with a CD4 count of above 100 cells/mm³; hence, the CD4 count value below 100 cells/mm³ is currently used in excluding people living with HIV from the shorter regimen. For people living with HIV with a CD4 count above that threshold, both regimens can be used.
Children living with HIV were eligible for enrolment in the SHINE trial. In view of the limited evidence available from the trial, clinicians may consider treating children living with HIV with non-severe TB for 4 months with 2HRZE/2HR, depending on the degree of immunosuppression and ART status, as well as the presence of other opportunistic infections. These children and adolescents will need to be monitored closely, especially at 4 months of treatment.
As discussed above, all people living with HIV (especially those with TB) should receive ART. People with HIV-associated TB who are responding to ART should not expect a less favourable outcome to a treatment episode than those who are HIV-negative. Therefore, people living with HIV with DS-TB can benefit from currently recommended treatment regimens. For further information, see WHO’s The use of antiretroviral drugs for treating and preventing HIV infection (22), WHO consolidated guidelines on tuberculosis: Module 6: tuberculosis and comorbidities, second edition (37) and WHO operational handbook on tuberculosis: module 6: tuberculosis and comorbidities (38).
6.3 Considerations for implementation
There are no new implementation considerations beyond the current standards of care for people living with HIV. NTPs need to work closely with HIV programmes to further expand HIV testing and ART coverage among TB patients. A particular exception highlighted in the recommendation on timing of the ART relates to situations when signs and symptoms of meningitis are present. In general, it is recommended to start ART within 2 weeks of initiating TB treatment; however, caution is needed in people living with HIV with tuberculous meningitis, because immediate ART is significantly associated with more serious AEs. Thus, delaying ART for 4–8 weeks after initiation of TB treatment might be considered in these situations (39). In patients commencing ART with a CD4 count of less than 100 cells/mm3, while steroids may reduce TB-related IRIS, more data are needed on their use in preventing IRIS in patients with low CD4 counts.
6.4 Treatment monitoring
There are no new monitoring and evaluation considerations beyond the current standard of care for people living with HIV. In view of the subgroup considerations, NTPs may consider monitoring specifically for relapse in this group of TB patients. More details on treatment monitoring are given in Section 9.

 Обратная связь
Обратная связь