Перекрёстные ссылки книги для 1266
Introducing the longer and shorter MDR-TB regimens entails a series of steps that are the same as those necessary when an NTP introduces a new MDR-TB treatment component. This section summarizes some key considerations for those steps.
9.1 Policy and operational documents
Policy and operational documents that govern the main components of the programme will need to be revised. Such documents include the national strategic plan for TB, treatment guidelines and algorithms, diagnostic algorithms, the essential medicines list, regulations (e.g. importation of clofazimine and pretomanid), drug orders and training material. Ideally, the type of regimen used by the patient would be indicated in the register and could be summarized for reporting. The TB treatment card may be changed to allow the tabulation of results of periodic testing for treatment response and adverse reactions (this may have already been done for the purposes of aDSM) (16). Any changes should also cover the use of the regimen in private practice.
9.2 National DR-TB expert committee or technical working group
A national MDR-TB expert committee or technical working group (the consilium or its equivalent structure within the NTP) will assist health care providers as early as possible to:
- coordinate policy changes and activities related to the introduction of the revised MDR-TB regimens in both the public and private sectors (e.g. training, communication and establishing patient eligibility for the different MDR-TB regimens);
- train staff in the clinical aspects of aDSM;
- provide patient support; and
- provide technical and clinical advice.
Additional support may be provided by other experts at national and international levels, and at regional level through, for example, the regional Green Light Committee (rGLC). In making use of such support, it is important to consider any phased implementation process, such as the initial introduction in one or a few centres before full scale-up, or whether implementation is also occurring in the private sector.
9.3 Electronic recording and reporting
There is a need to improve the quality of patient data using standardized variables, such as data on DST patterns, prescribed treatment, treatment outcomes and adverse drug reactions. The collection and utility of these data are important for future evidence-based recommendations, especially given the lack of RCTs on the management of DR-TB (139). If digital patient records do not already exist, it is important that the programme managers consider their introduction, at least for surveillance and possibly also for case management (182). If patient records are already digital, changes may be needed in the electronic recording and reporting system to allow individuals belonging to MDR-TB regimen cohorts of interest (e.g. shorter regimen, bedaquiline-containing regimens and operational research subgroups) to be identifiable, and for certain options to be included in the monitoring framework (e.g. addition of clofazimine and registration of ECG findings). It is crucial for programmes to maintain such data diligently and prospectively, so that they can contribute to programme evaluation and to global policy-making (e.g. the development of the WHO consolidated guidelines benefited hugely from the experience of patient treatment within programmes) (136, 137). The treatment outcome cohort reports for MDR/RR-TB do not need to change (for the digital and paper version). Moreover, electronic tools can enhance the quantification of consumables; for example, volumes of medicine can be calculated automatically using QuanTB, an application (app) that is available for download free of charge.²⁵ It is important to ensure that digital records can accommodate key measures in children that may differ from those for adults.
9.4 Estimates (epidemiological and logistics)
Estimates are needed by the NTP and other health care providers, to determine the number of MDR/RR-TB patients eligible for the longer and shorter MDR/RR-TB regimens, to revise the budget accordingly, and to submit the corresponding requests for drug orders, taking into account the existing stock of medicines. These estimates of MDR/RR-TB patients likely to be enrolled are based on current notification trends and an expected increase in line with national and subnational plans. The programme first establishes the number of MDR/RR-TB enrolments expected in the coming years, depending on the future increase in programme capacity (e.g. as part of a project supported by a grant from the Global Fund to Fight AIDS, Tuberculosis and Malaria). Then, based on knowledge from surveillance, eligibility and estimated rate of scale-up, the programme defines different patient groups; for example, those expected to receive different variants of the longer MDR/RR-TB regimens and those likely to receive a shorter MDR/RR-TB regimen. When estimating the caseload to put on treatment, it is necessary to factor in not just eligibility, but also what would be feasible to achieve within a given time, to ensure that all elements are in place for starting and maintaining patients on treatment (e.g. training and provision of an adequate framework for patient monitoring and support). Associated programme and patient costs other than the medicines themselves usually dominate the total cost for both longer and shorter MDR/RR-TB regimens (e.g. treatment of AEs, hospitalization, diagnostic consumables, other clinical care and social support); however, total costs are expected to be lower for shorter regimens, given the shorter duration of treatment.
9.5 Management of the supply chain and storage conditions for pharmaceuticals
Management of the supply chain and storage conditions for pharmaceuticals should be reviewed to ensure that TB drug orders are made in good time and are correctly quantified to avoid overstocking or shortages. The NTP must ensure an uninterrupted supply of TB medicines through proper quantification, supply planning and rigorous quarterly monitoring, with a functional early warning system to avoid stock-outs and subsequent treatment interruptions. Similarly, other consumables (e.g. medicines for symptomatic relief and adverse reactions, syringes, diagnostic kits, medication for management of adverse effects, masks and N95 respirators) will be needed to ensure that the intervention is delivered as per internationally recommended standards (183). The principles for the quantification of medicines needed for the longer and shorter MDR/RR-TB regimens are similar. The health care provider needs to have some basic details about how many patients will be treated and when they will start; the expected increment in caseload over successive years; the average body weight of the patients; whether children will also be enrolled; the expected losses (from interruptions, deaths and transfers to another regimen); and current stock on hand, including expiry dates and orders of medicines already in the pipeline and not yet delivered. It is best to split an order of medicines, the first part being for the patients expected to be started within 6 months, and the second part adjusted based on the actual enrolments. Technical assistance to strengthen the procurement and supply and to establish an early warning system for stock-outs can be accessed via the secretariats of the Global Drug Facility (GDF) and rGLC (which are housed in WHO regional offices) or via the WHO country offices. GDF provides support to many NTPs on the procurement and supply chain aspects of phase-in and phase-out plans of products or regimens and can procure child-friendly formulations. Child-friendly formulations allow more accurate paediatric dosing and are more acceptable to children and parents; they should be provided as the SoC wherever possible.
9.6 Preparation for the introduction of new treatment regimens
When planning important changes for the national TB treatment policy to align with the latest WHO recommendations, the NTP needs to balance the will to provide the best possible options for patients according to the latest evidence against the programmatic circumstances and the implications of such changes (e.g. the need to re-train staff or obtain funds for reprogramming). The programme must balance the need to provide access to new medicines for which the evidence is still incomplete with the need to protect patients from avoidable toxicity, the emergence of resistance to the new agents and observance of proper ethical conduct and respect for patient rights.
Given the increased use of newer and repurposed medicines in combination MDR-TB regimens, aDSM is particularly important. aDSM defines the active and systematic clinical and laboratory assessment of patients on MDR-TB treatment to detect, manage and report suspected or confirmed drug toxicities (16). It applies the principles of active pharmacovigilance to the specific needs and context of NTPs, and is embedded within the routine patient monitoring function (e.g. treatment outcome cohort monitoring) of NTPs. The management of patient safety is an inherent part of aDSM, inseparable from its monitoring component. The recording and reporting activities of aDSM primarily target serious AEs as a priority requirement, but any AE during treatment administration (whether or not it is related to drug toxicity) needs to be managed to limit harms to patients. MDR-TB treatment sites may also monitor nonserious AEs that are of clinical significance or of special interest to the programme, as part of more comprehensive aDSM. In aDSM, in addition to the spontaneously reported reactions, AEs are elicited as part of a patient monitoring plan that comprises a set of questions and often an array of laboratory or clinical tests at defined periods of time, before, during and after treatment (Annex 3).
To ensure rapid uptake of the WHO-recommended shorter treatment regimens for TB, NTPs should take the lead on gathering a technical working group (TWG) from the representatives of affected communities and partners organizations to develop an introduction plan that defines all necessary measures and organizations responsible for implementation. Subsequently, the TWG, in collaboration with NTP, needs to coordinate the practical implementation of the planned activities. Table 2.9.1 presents a checklist of activities for considerations by the programme manager when planning and implementing the DR-TB regimens that are newly recommended.
For the successful introduction of a new regimen, the following needs to be achieved at the different levels of the NTP.
National level:
- Updated regulatory approval of the new regimen and the updated national policy;
- Funding available for all necessary steps and requirements; note that eventually, the use of the new regimen may lead to reduced costs;
- Tools for the nationwide introduction of the new regimen are available (e.g. updated national guidelines, training of trainers and training materials for all relevant cadres, needs assessment of clinical and laboratory services, funded nationwide scale-up plan);
- Updated surveillance and monitoring and evaluation system (including active drug-safety monitoring and management [aDSM] if required), revised treatment outcome indicators;
- An adequate drug procurement system, with the proper quantification and forecasting of new drugs for the new regimen; and
- Partnership with clear roles and responsibilities (Ministry of Health [MoH]/NTP, technical partners, donors, patient organizations).
Health facility level in adopting areas
- Clinical Expert Committee/Consilia oriented on the updated/new guidelines and role ensured, especially the provision of clinical advice;
- Personnel are trained in clinical and programmatic implementation of the new regimen, including aDSM (if required), and roles and responsibilities clearly laid out;
- Facilities at the institutional level using updated national guidelines, and meeting requirements of the new regimen (e.g. people-centred approach, social support in place);
- Access to the relevant laboratory (clinical and bacteriological) tests is secured, and facilities equipped with the tools for conducting of clinical assessment for AEs (e.g neurological examination, visual acuity and colour vision tests, ECG, etc.); and
- The surveillance system, programmatic standard operating procedures (SOP) and patient management protocols are adequately implemented.
Table 2.9.1. List of activities and corresponding responsible organizations
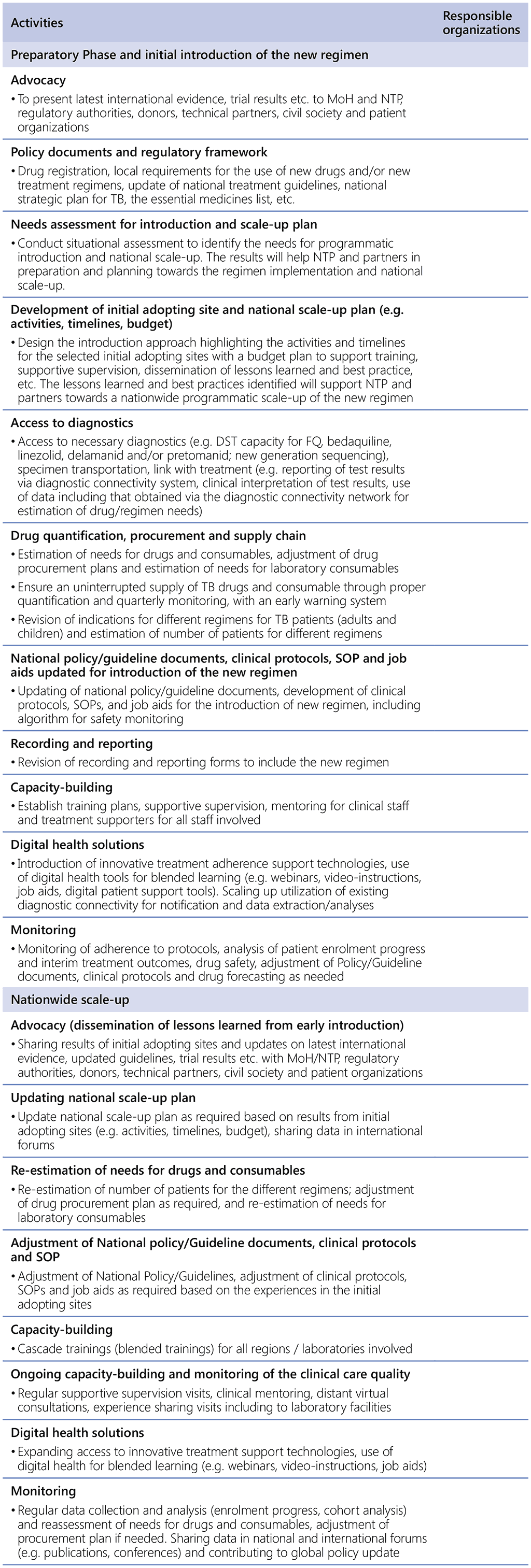
Costing
Funding sources for the introduction of the new regimen should be identified and funds secured at the time of preparation. In addition to the expected cost of planned activities, the confirmed or possible sources of funding should be noted in the budget plan. This will allow for targeted fund raising and advocacy to ensure that the costs of implementation will be covered. The budget should include the following items:
- Costs of medicines and pharmaceutical products (e.g. component drugs in the new regimen and ancillary drugs);
- Supply and management costs (procurement, storage, distribution);
- Costs of preparation activities (e.g. task force meetings, TWG meetings, sensitization workshops, situational assessment, communication materials, development of national plan, administration, technical assistance);
- Costs of system strengthening to meet the minimum requirements (e.g. upgrading/renovating infrastructure, maintenance, equipment needed for laboratory and for patient monitoring including for aDSM such as ECG, tuning forks, Ishihara test), costs of referral to laboratories and specialists;
- Costs of implementation (e.g. human resources including non-TB specialists, technical and management assistance, printing of materials, including job aids, training, laboratory reagents and consumables costs, aDSM, supportive supervision and monitoring visit to implementing sites, patient transport, or incentives); and
- Costs for enhanced monitoring and evaluation (M&E) during the initial adopting sites period and technical assistance for analysis and publication and dissemination of results in local, national, regional and international forums.
Monitoring and evaluation
Recording and reporting of the use of the new regimen should be part of the routine surveillance system established at country level for programmatic management of TB, including:
- Standardized definitions of cases and treatment outcomes;
- Standardized registration of cases;
- Generation of interim results and treatment outcome indicators; and
- Generation of data on the occurrence of serious AEs.
All the above need to adhere to the latest WHO recommendations and definitions.
Information on enrolment, results of treatment and AEs for all patients diagnosed and treated in the initial adopting sites, and subsequently in all sites of the country, should be collected; all patient related data should be disaggregated for sex and age groups (children 0–4, 5–14, adults). This will allow for assessment of experiences of patients and health workers, the implementation of the diagnostic and treatment algorithms (triage) and will allow for assessment of treatment outcomes in either cohort of DS-TB or DR-TB patients receiving different treatment regimens.
To ensure appropriate introduction of the new treatment regimen, a schedule for regular on the job support needs to be realized. National M&E protocols need to be adjusted for the new treatment regimen and used to generate the required information for the country to fine tune its implementation model.
The experiences of the initial adopting sites should be documented by collecting key data related to M&E results and programmatic implementation to inform the subsequent nationwide expansion of the new regimen. The interim cohort review will also allow to evaluate treatment response of individual patient during treatment and take timely action at patient and programmatic levels to prevent unfavourable treatment outcomes.
9.7 Monitoring treatment response and safety
Individuals undergoing MDR/RR-TB treatment require close monitoring throughout treatment, using appropriate clinical and laboratory testing schedules. Regular assessments should include medical history reviews, physical examinations, specialized tests (e.g. visual acuity assessments, peripheral neurological exams and electrocardiography) and laboratory monitoring (Table 2.9.2).
9.7.1 Monitoring the treatment response
The treatment response should be monitored through monthly sputum smear microscopy and culture, ideally conducted at the same frequency. Regular clinical assessments are critical to evaluate improvements in TB signs and symptoms, assess regimen effectiveness and prevent LTFU.
It is not always necessary to repeat radiological assessment during treatment because some radiological abnormalities may persist throughout and beyond treatment completion; hence, they may not indicate poor response or failure of treatment. However, radiological deterioration and new abnormalities (compared with baseline) may assist in identifying a poor treatment response. Therefore, radiological assessment should be repeated if clinically indicated.
There is growing evidence of resistance to both bedaquiline and linezolid in Mtb strains, particularly in patients who have been previously exposed to these medications. Reliable DST for bedaquiline, linezolid and all medicines included in the treatment regimen is crucial to understand the reasons behind a lack of bacteriological and clinical improvement. Ideally, DST for all medicines used in regimens should be accessible. However, this must not delay the initiation of life-saving treatment.
Before beginning a 9-month regimen, it is essential to have the patient’s bacteriological status, including confirmation of TB disease, MDR/RR-TB (at a minimum) and FQ susceptibility. FQ DST is also critical for tailoring appropriate 6-month regimens such as BPaLM/BPaL, BDLLfxC, BDLLfx or BDLC. This ensures efficacy while minimizing unnecessary toxicity. However, the lack of immediate FQ DST should not hinder the initiation of treatment, because regimens can be started with combinations that allow flexibility pending FQ DST results.
NTPs should prioritize establishing baseline DST capacity for bedaquiline and linezolid, especially in patients with FQ resistance. Additionally, DST should be conducted on samples from patients with no bacteriological conversion after 4 months of treatment or in cases of recurrence while using 6-month regimens. Collecting strains for sequencing should also be considered as part of this effort.
All patients, and particularly children, should be followed up for clinical reassessment beyond treatment completion (ideally over a 12-month period) to monitor for potential relapse.
Treatment monitoring for special populations
For children, monitoring should also include regular assessments of weight, height and BMI using age-appropriate growth charts. Radiological assessments are not routinely required in children, but may help to identify poor treatment response in cases of clinical deterioration or when new abnormalities arise compared to baseline.
Monitoring treatment in pregnant and breastfeeding women with MDR/RR-TB requires close attention to both safety and efficacy; for this population, the 6-month regimen BDLLfxC or 9-month regimens, BLMZ or a linezolid variation, may be used (see Section 5). The modified 9-month regimen, BLMZ, is preferred over the 9-month regimen with linezolid owing to its limited drug count and history of use in pregnancy. Regular assessments should include fetal development monitoring, maternal health checks and drug-specific AE screening.
For breastfeeding women treated with shorter regimens, extra vigilance is needed to monitor infant health and potential drug exposure through breast milk. In both cases, adherence support and proper administration of MDR/RR-TB treatment are crucial. Care providers must ensure continuity of care between antenatal and TB services, which are often poorly integrated in high TB burden settings. Good communication between providers and the patient is critical to avoid unnecessary treatment modifications, reduce stigma and prevent LTFU.
For PLHIV, there are limited data but some promising results from current research. Monitoring should focus on regular viral load and CD4 count assessments, vigilant screening for DDIs between TB medications and ART, close observation for overlapping toxicities, and adherence support for both TB and HIV treatments.
Treatment outcome definitions and reporting frameworks for DR-TB regimens, whether short or long, are consistent with those used for DS-TB regimens (see Section 10, Chapter 1).
9.7.2 Monitoring safety
Active surveillance for AEs is essential for all DR-TB regimens, to ensure patient safety and minimize both short-term and long-term morbidity. The WHO framework for aDSM (Annex 3) should be applied to patients receiving DR-TB regimens.
The recommended schedule for baseline, routine and post-treatment monitoring examinations (Table 2.9.2 and Annex 2) applies to all DR-TB regimens, including 6-month, 9-month and longer regimens. This guidance ensures comprehensive patient monitoring, including monitoring of the treatment response and safety. The schedule should also consider specific situations where more frequent assessments may be necessary; for example, in cases of electrolyte disturbances, haematologic abnormalities or ECG irregularities. Additionally, closer monitoring is advised for high-risk groups, including older adults; PLHIV; and people with hepatitis caused by HBV or HCV, diabetes mellitus, moderate to severe hepatic or renal impairment, baseline anaemia or visual disturbances (e.g. glaucoma, cataracts or colour blindness). Audiometry and specific biochemical tests should also be made available whenever certain agents are included in regimens.
Generating additional evidence on AEs will further strengthen the safety profile of the shorter regimens across a variety of implementation settings. NTPs should actively monitor drug safety to ensure proper patient care, report AEs to the relevant drug-safety authority within the country, and contribute to informing both national and global policy
Table 2.9.2. Recommended schedule of baseline, routine and post-treatment monitoring examinations for patients on DR-TB treatmentᵃ
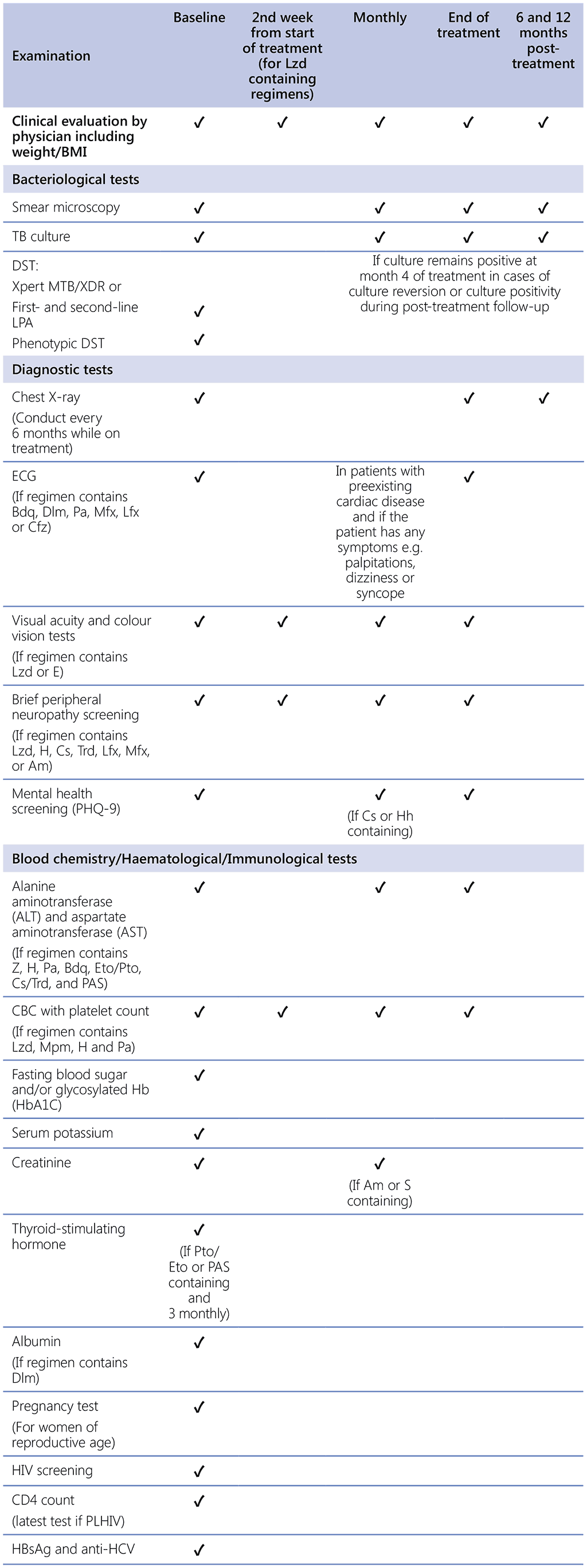
BMI: body mass index; CBC: complete blood count; DR-TB: drug-resistant TB; DST: drug susceptibility testing; ECG: electrocardiogram; Hb: haemoglobin; HBsAg: hepatitis B surface antigen; HCV: hepatitis C virus; HIV: human immunodeficiency virus; LPA: line probe assay; MDR/RR-TB: multidrug- or rifampicin-resistant TB; PHQ-9: Patient Health Questionnaire; PLHIV: people living with HIV; TB: tuberculosis.
Drugs: Am: amikacin; Bdq: bedaquiline; Cfz: clofazimine; Cs: cycloserine; Dlm: delamanid; E: ethambutol; Eto: ethionamide; H: isoniazid; Hh: isoniazid high dose; Lfx: levofloxacin; LPA: line probe assay; Lzd: linezolid; Mfx: moxifloxacin; Mpm: meropenem; Pa: pretomanid; PAS: p-aminosalicylic acid; Pto: prothionamide; Trd: terizidone; Z: pyrazinamide.
a Schedule is applicable to all the recommended MDR/RR-TB treatment regimens.
9.8 Treatment outcome definitions
The standardized treatment outcome definitions are summarized in Section 10, Chapter 1. The treatment outcome definitions allow all patients with either DS-TB or DR-TB to have a treatment outcome assigned when completing treatment (cure or treatment success) or when unfavourable events occur (e.g. LTFU, failure or death). Although the definitions of treatment outcomes have been harmonized since 2020, minor differences remain between those for DS-TB and DR-TB (e.g. treatment monitoring by sputum culture for DR-TB and by sputum smear microscopy for DS-TB) (184). Although the role of new bacteriological tests was considered, treatment monitoring will continue to rely on the available tools (i.e. sputum culture for DR-TB and sputum microscopy for DS-TB), despite their limitations.
9.9 Post-treatment follow-up
Patients that have completed a DR-TB regimen with a successful treatment outcome, ideally need to be monitored for recurrence for a duration of at least 12 months after the end of treatment. Some programmes may have capacity to ensure post-treatment follow-up and record the additional, optional outcome that is described in the WHO standard treatment outcome definitions (184).
A patient who presents with TB after treatment completion may be sick due to a relapse with the same TB strain or due to reinfection with a new strain. Genetic fingerprinting can compare the initial infecting TB strain to the recurrent TB strain. In many settings it is not possible because genetic fingerprinting is not available. In areas with access to genetic fingerprinting, documenting relapse from reinfection can be a piece of important evidence to guide the design of the retreatment regimen. If programmes have the capacity, they are encouraged to freeze the baseline specimen so that it can be used for future comparison should the patient relapse or acquisition of drug resistance is the reason for treatment failure or relapse.
Patients in whom a DR-TB regimen has failed to produce a successful outcome present unique challenge and often their next regimen may be their last opportunity for cure.
This handbook suggests the following post-end-of-treatment schedule, described in Table 2.9.3. Monitoring cultures are sent at month 6 and month 12 regardless of if the patient has symptoms of TB or not. A combined monitoring table for post-end-of-treatment follow-up is provided in Table 2.9.3.
The patient should be well informed to contact the programme should symptoms of TB reoccur or if any family members or close contacts develop symptoms of TB. At the end of 12 months of post-end-of-treatment follow-up, the patient will be given a post-end-of-treatment outcome: sustained successful treatment (no evidence of recurrence), recurrence, death or lost to follow-up.
Table 2.9.3. Post-end-of treatment schedule
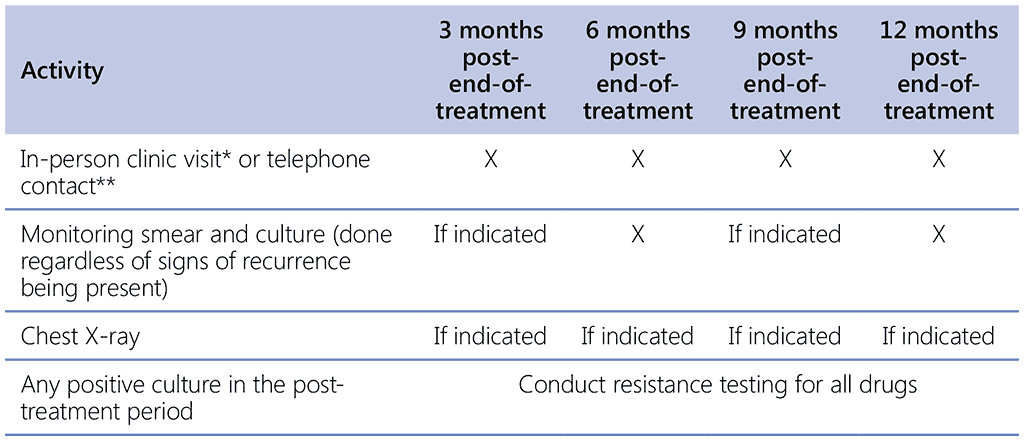
*The clinical visit should consist of the weight, BMI, and brief peripheral neuropathy screen as well as any other clinical and laboratory examination needed based on symptoms.
**Telephone contact is acceptable only when it is not feasible for the patient to come for an in-person clinic visit. Discuss directly with the patient only, unless permission has been granted to discuss the patient’s illness with a family member or health proxy.
25 Available at https://siapsprogram.org/tools-and-guidance/quantb/.

 Обратная связь
Обратная связь