Enlaces transversales de Book para 1266
TB infection is a state of persistent immune response to Mtb antigens with no evidence of clinically manifest TB disease (35). People with TB infection have no signs or symptoms of TB disease, are not infectious, have normal or stable images on chest X-ray (CXR), and have negative microbiological tests (if such tests are performed). It is estimated that about 25% of the world’s population has been infected with Mtb (1), of whom 5–10% will develop TB disease over their lifetime (36). This risk is much higher in those with certain epidemiological factors (e.g. recent contact with bacteriologically confirmed pulmonary TB), social characteristics (low socioeconomic status or malnutrition) and demographical characteristics (e.g. very young children or the elderly) or clinical conditions that compromise the immune system (e.g. HIV infection, diabetes or immunosuppressive medications) (37–39).
Among people with such risk factors, TPT can provide important individual and public health benefits. However, implementing TPT raises various challenges in terms of programme prioritization, reluctance of health workers to treat people who are asymptomatic, medication adherence, availability of appropriate drugs and formulations, costs, demands on health systems and the individuals concerned, and access to free screening and testing for TB infection. TPT can induce adverse drug reactions (although these are rarely serious) in people who are generally healthy. Hence, TPT is recommended only for groups who are at high risk of developing TB disease, in whom the benefits of TPT clearly outweigh the risks.
The risk of TB disease is higher in those who have a positive test for TB infection than in those with the same risk factors but a negative test for TB infection. In addition, it is useful to test for TB infection because individuals who test positive are more likely to benefit from TPT than those who test negative; thus, TPT will be of greatest individual and public health benefit if it is directed by an assessment of risk alongside the results of testing for TB infection. Hence, the extension of TPT creates a need to expand the capacity to test for TB infection. However, the absence of TB infection testing should not prevent the use of TPT, particularly for TB contacts aged below 5 years and people living with HIV (41).
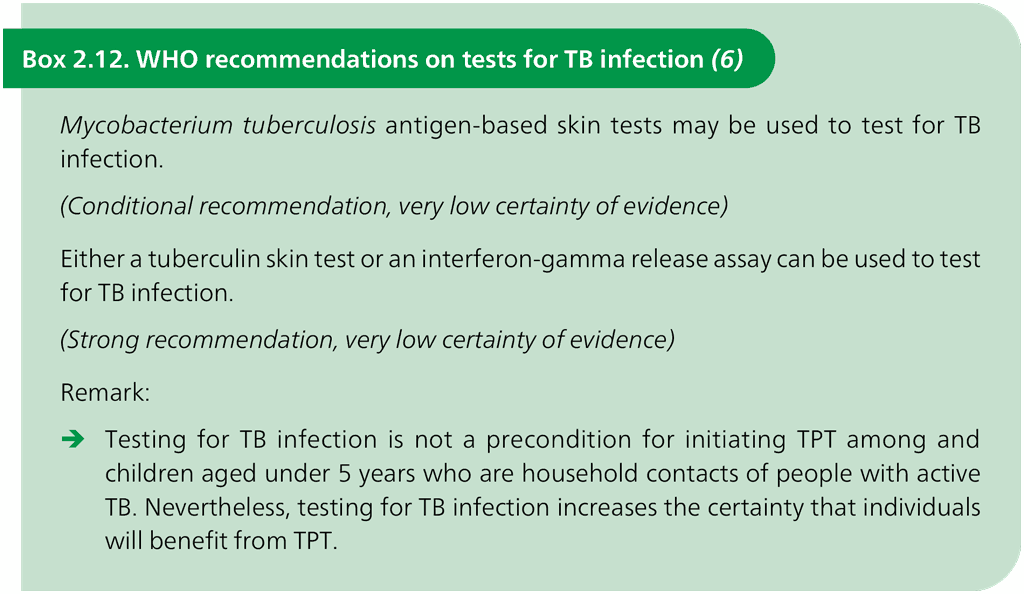
WHO recommends three classes of tests for TB infection: TST, TBSTs and IGRAs (Box 2.12). These tests are described in more detail in the subsections below.
2.6.1 The TB infection cascade of care
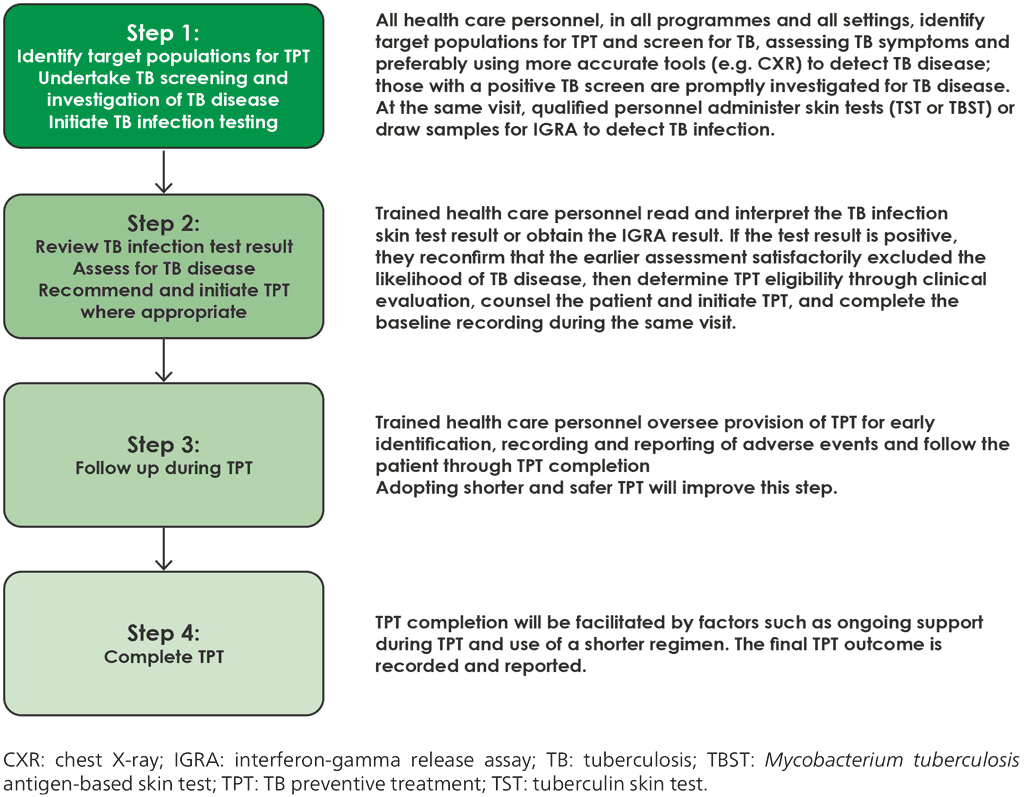
Identifying, testing, evaluating and treating people with TB infection is a multistep process that has been termed the “TB infection cascade of care” (40). A systematic review showed that these losses resulted in less than 20% of those eligible for TPT completing their course of medication (40). Importantly, under field conditions, 70–80% of the losses occurred before the initiation of TPT. Various practical and programmatic challenges limit access to testing for TB infection. The impact of these challenges on the cascade of care must be considered at local level, and steps taken to resolve identified challenges. A simplified four-step process is shown in Fig. 2.3.
Fig. 2.3. Simplified four-step person-centred TB infection cascade of care
Who should be tested for TB infection?
The decision to test an individual for TB infection implies an intention to offer TPT. Therefore, testing for TB infection should be reserved for populations in whom the risk of developing TB disease is high and who will benefit the most from TPT. Decisions to start TPT should always consider the risk of adverse drug events, in addition to TB symptoms and the results of the test for TB infection. Box 2.13 summarizes the groups WHO recommends should receive TPT, with testing not mandatory for Groups 1–5. More detail can be found in Module 1 of the WHO operational handbook on TB (41).

2.6.2 TB infection skin test using tuberculin
The standardized preparation of tuberculin from Mtb, termed “purified protein derivative (standard)”(PPDS), was first produced in 1941 by Florence Seibert (42); since then, all production of tuberculin material has used the same methods and testing against this standard. All commercially available tuberculin material, other than the “next-generation” skin test described later, are manufactured to produce PPD material that is bioequivalent to this standard PPDS.
PPDS contains a mix of antigens, including some that are specific to Mtb, but also many that are found in NTM and BCG. Hence, false positive reactions to PPDS have been described in people with NTM disease or with sensitization to NTM antigens (43), and in people who have received BCG vaccination, particularly if they received BCG more than once or after infancy (44).
Testing with PPDS is safe. Although severe local reactions with blistering can be seen in 2–3% of people, these are true positive reactions that are self-limited and heal spontaneously. Allergic reactions with generalized rash occur in less than 1% of people (45), and anaphylaxis occurs in only one person per million (46). Based on decades of experience, TST with PPDS is considered safe in pregnant and lactating women (47). A detailed step-by-step description on how to perform the TST test is provided in Annex 4.
2.6.3 TB infection skin tests using Mtb-specific antigens
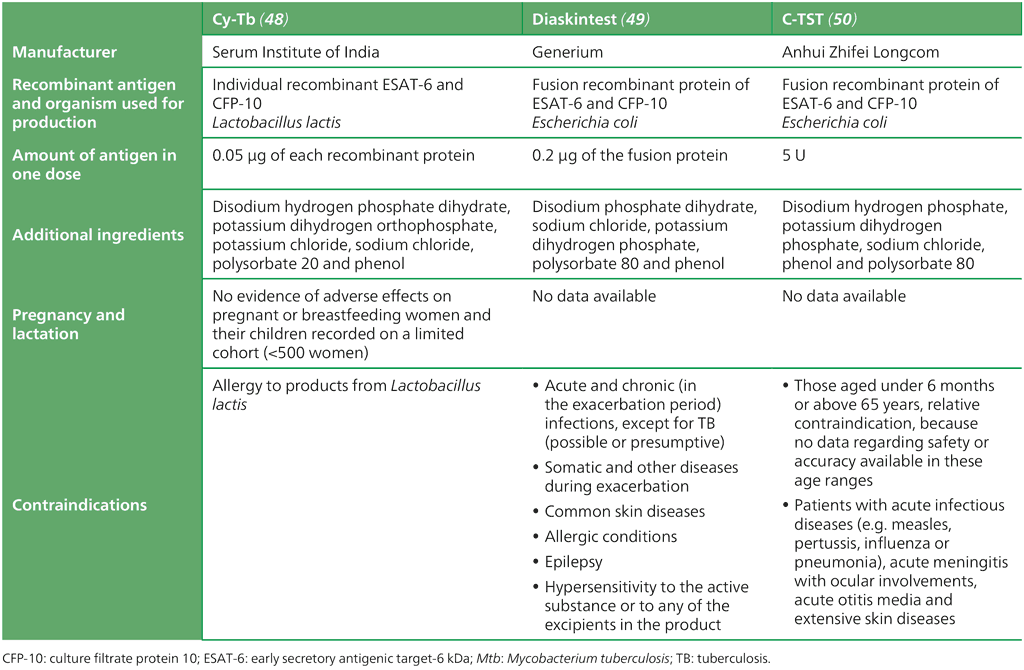
To make the in vivo skin testing more specific, various test manufacturers have developed skin tests with recombinant Mtb antigens. The Mtb-specific ESAT-6 and CFP-10 were used either as individual proteins or in one fusion protein combining the two antigens. WHO has assessed three tests that all showed acceptable performance and could be recommended for use. All tests are based on the same principle as the TST; that is, an intradermal injection of the recombinant antigens is made and induration is measured after 48–72 hours. A summary of the different tests recommended by WHO is found in Table 2.12. Of note, as of early 2025, the Cy-TB TBST was available through the Stop TB Global Drug Facility for only $1.50 USD per test; supporting low unit-cost implementation of TB infection testing programmes. Further details on the tests, procedures and interpretation of test results for TST and the three TBSTs are available in Annex 4.
Table 2.12. Comparison of Mtb-specific infection skin tests
2.6.4 Interferon-gamma release assays
IGRAs are in vitro blood tests that measure interferon-gamma released by circulating lymphocytes in whole blood during overnight incubation with exposure to Mtb-specific antigens (enzyme-linked immunosorbent assay [ELISA] based) or the number of T-lymphocytes producing interferon-gamma (enzyme-linked immunosorbent spot [ELISPOT] based). In 2011, WHO issued its first recommendations on the use of IGRAs for the diagnosis of TB infection; three tests were included in this class. Following the advice of the TAG held in January 2025, WHO updated the list of tests that meet the class criteria, supported by policy statements on their use (Box 2.14). The tests included in the class for which the WHO recommendations apply to are:
- Qiagen’s QuantiFERON-TB Gold Plus;
- Oxford Immunotec T-SPOT®.TB;
- Beijing Wantai’s TB-IGRA;
NEW
- SD Biosensor’s STANDARD E TB-Feron ELISA; and
- Diasorin’s LIAISON QFT-Plus (CLIA).
QuantiFERON Gold and QuantiFERON Gold In-Tube were previously recommended by WHO; however, they have been discontinued by the manufacturer.

The five tests can be grouped into three types of IGRA detection methods:
- ELISAs (QFT-Plus, Wantai TB-IGRA, STANDARD E TB-Feron)
- ELISPOT (T-SPOT.TB) (52)
- CLIAs (LIAISON QFT-PLUS)
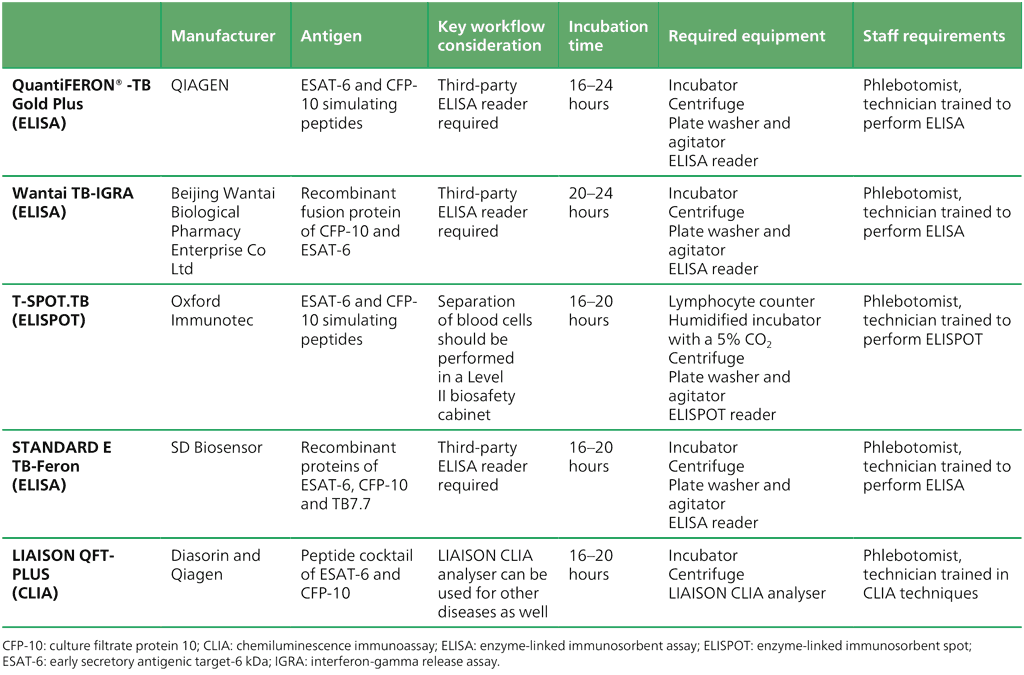
In contrast to TST and TBST, the WHO-recommended IGRAs require a well-equipped laboratory and trained laboratory technicians. Similarly to the recommended TBSTs, IGRAs are based on the lymphocyte response to Mtb-specific antigens (ESAT-6 and CFP-10), meaning that results are not affected by prior BCG vaccination. However, challenges with phlebotomy in young children limit the applicability of IGRAs, with skin tests being the alternative. A comparison of the different IGRA tests is given in Table 2.13 (which covers all tests) and in Web Annex D (which covers only new within-class products). The guidance provided should facilitate the procurement and uptake of the recommended technologies and improve patient care.
All products recommended by WHO are automatically eligible to be included in the WHO essential diagnostic list. The WHO recommendations on diagnostics are based on clinical research evidence; they do not include quality assessments of the products or the manufacturing process involved. Before introducing any new products, countries should ensure that those products fulfil local or internationally recognized regulatory requirements.
Table 2.13. Comparison of IGRA tests

 Reacción
Reacción