Enlaces transversales de Book para PLATAFORMA DE INTERCAMBIO DE CONOCIMIENTOS SOBRE LA TB DE LA OMS
This annex provides step-by-step procedures for administering and reading two types of skin test for tuberculosis (TB) infection: the tuberculin skin test (TST) and Mycobacterium tuberculosis antigen-based skin tests (TBSTs). The photographs used in this annex were kindly provided by Dr Richard Menzies and colleagues.
A1 – Test administration
Step 1. TB Screening check
In an integrated and person-centred TB infection cascade of care, the first encounter with a provider will include initiation of both screening for TB disease and testing for TB infection. Just before administration of the TST or TBST, TB disease screening should be done; it should include a TB symptom check and preferably where available use more sensitive tools recommended by WHO such as chest X-ray (CXR, with or without computer-aided detection), WHO-recommended rapid diagnostics or C-reactive protein for people living with HIV. If a contact has clinical features that suggest TB disease, then that person should be referred immediately for further diagnostic evaluation, which should be done on the same day.
Where a contact has no symptoms or has only non-TB-related respiratory symptoms (e.g. a sore throat or rhinorrhoea, with a duration of only a few days), then TB infection testing can be initiated, and these symptoms can be re-evaluated 48–72 hours later. It is important to aim to minimize loss along the TB infection care cascade. The decision to immediately evaluate an individual with symptoms, or to perform TB infection testing and re-evaluate symptoms at the time of reading, is a matter of clinical judgement.
Step 2. Explain the administration of TST or TBST
Signed informed consent is not considered necessary for TB infection testing because this is part of routine care; however, the person being tested should understand the procedures and agree to them. In cases where a child is administered the test, consent by a parent or guardian is required and the presence of the parent or guardian may be necessary. The provider should take time to explain the test procedures and emphasize the need to return for test reading within 72 hours. The provider should verify that the person can return within this time frame for the test to be read; the provider should also respect the individual’s confidentiality and privacy.
If an mHealth approach is being used to measure the size of swelling immediately after intradermal injection as a quality control (QC) procedure (mTST) (1) or similar) the provider should explain to the patient that photos will be taken of the injection site immediately after the injection.
Step 3. Prepare for the administration of TST or TBST
It is important that the person undergoing the TB infection test is comfortable while the test is being administered. The person should be seated with their arm supported on a flat surface, such as the corner of a table, facing the provider who will administer the TB infection test. Where possible, the TB infection test should be administered in a private room, with only the person being tested in attendance (family members are appropriate).
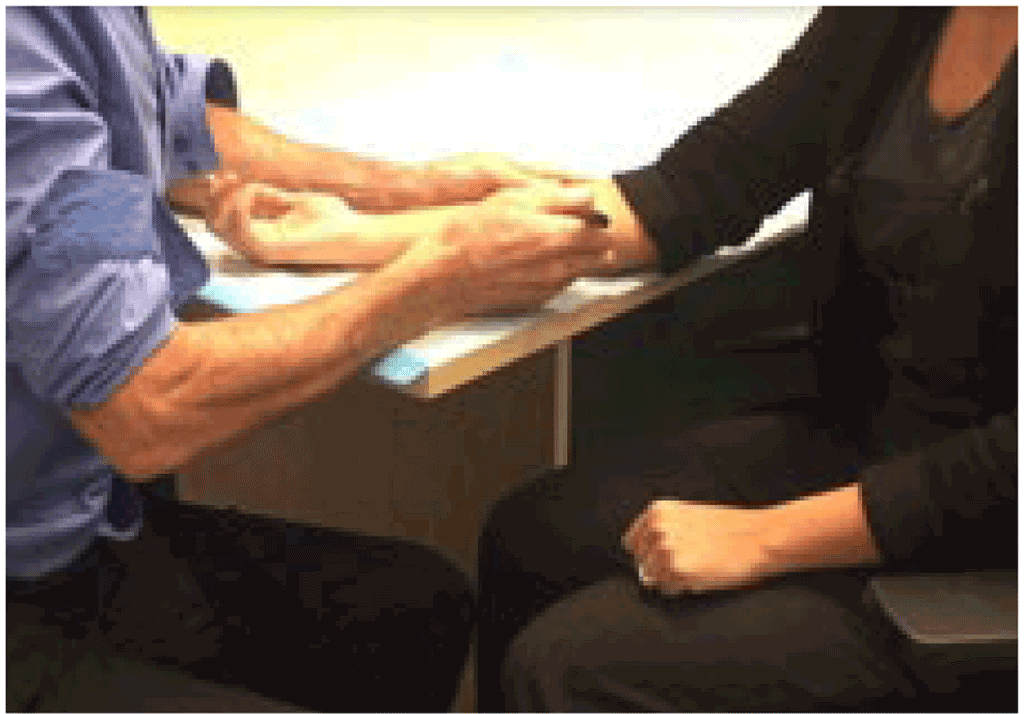
Preparing to perform the injection. © Richard Menzies
Step 4. Select site for injection
TST and TBST tests are generally administered on the inner aspect of the forearm, about 10 cm below the elbow (middle to upper third of the forearm). The area to be injected should be free of recent cuts, burns or other injuries, and not affected by a rash or eczema. The area should also be free of scarring, particularly keloids. Skin disease or scars may interfere with the injection and proper reading of the result; also, they may cause greater discomfort from the test. The presence of tattoos is not a problem unless erythema also needs to be measured (for C-TST only). If there are such skin problems, then another site should be selected for injection.
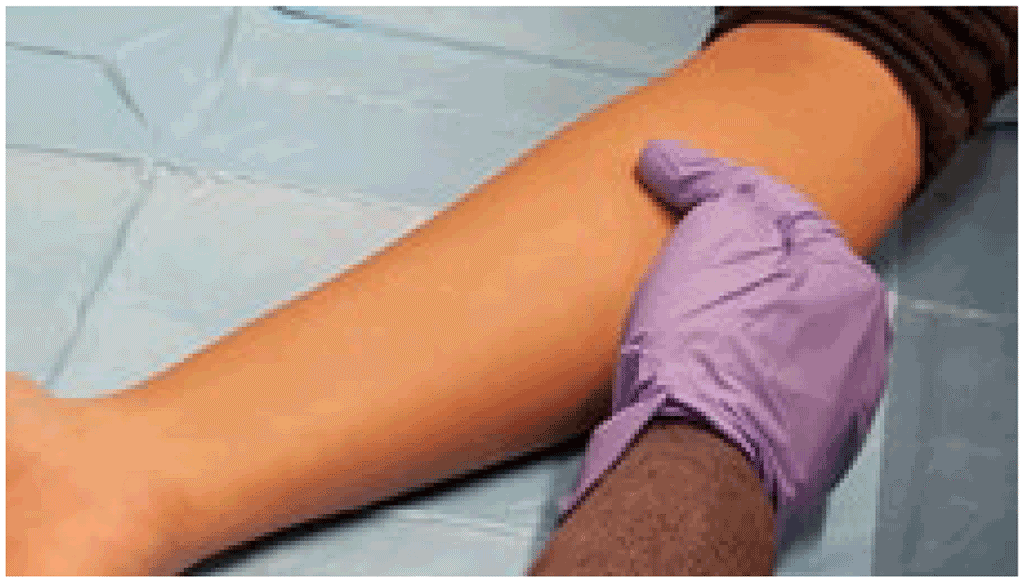
Adminster the TST or TBST 10 cm below the elbow. © Richard Menzies
Step 5. Prepare the syringe
Draw up the test dose in a 1 mL syringe that has markings to indicate each 0.1 mL (or smaller units). It is important to eliminate air from the syringe, to prevent accidentally injecting air into the patient and to ensure that the full test dose is administered. The syringe should be prepared just before the injection; older studies with standard purified protein derivative (PPDS) demonstrated reduced sensitivity if syringes were prepared more than 20 minutes before injection (2). If the mTST QC programme or similar is in place, the smartphone or digital camera should be prepared, so that the provider is ready to take photos immediately after the injection.
Step 6. Clean the injection site
Opinion varies on how best to clean the injection site. Alcohol swabs may be used, but this may cause greater pain unless the alcohol is allowed time to dry completely (it is important not to blow on the site, because this will reinfect the area). Allowing the alcohol to dry completely is especially important when administering the test to a child. Cleaning the area with sterile saline or water is adequate and will cause less stinging if the liquid has not fully dried at the time of injection.
A topical anaesthetic should not be applied, because this can sensitize the skin. There have been case reports of induration resulting from topical anesthetics (2), and that induration will be mistaken for a positive test at the time of reading.
Step 7. Administer the injection
All TB infection skin tests (TST or TBST) are administered using the Mantoux method of intradermal injection. This method of administration was first described for TST many decades ago (3), and the manufacturers of the three TBSTs have all described this same method in their product monographs (4–6). The material must not be injected subcutaneously because this makes it more difficult to measure the result and can even lead to false negative results.
A needle (25 gauge to 27 gauge) is placed on the syringe, which is then laid flat on the skin with the bevelled side upward. The provider then slides the needle under the skin (the tip of the needle is often visible even when it is intradermally). The material is then slowly injected (over 2–3 seconds). In cases where the test material runs out initially, the needle should be pushed in a little deeper. A small induration or papule or bleb (“weal”) should form. This bleb should be at least 7 mm in diameter (1). When the injection is finished, the needle should be withdrawn.
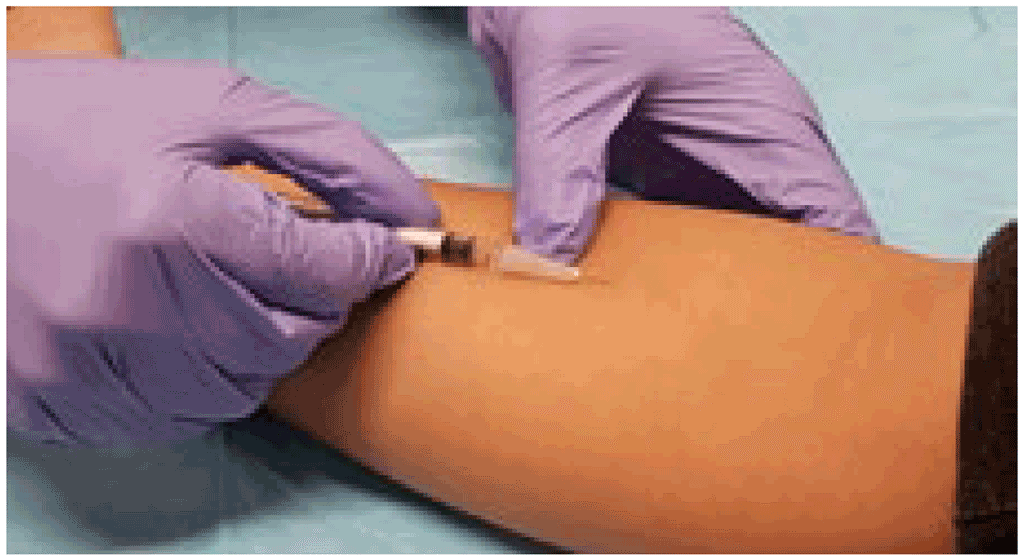
Perform an intradermal injection. © Richard Menzies
Step 8. After the injection
After the injection, there should be a small induration or bleb on the skin. If there is a little bleeding, this can be wiped away. There is no need to cover the injection site with a dressing or bandage, and no need to mark the spot (having a large circle or an X on the forearm may be stigmatizing, particularly for children).
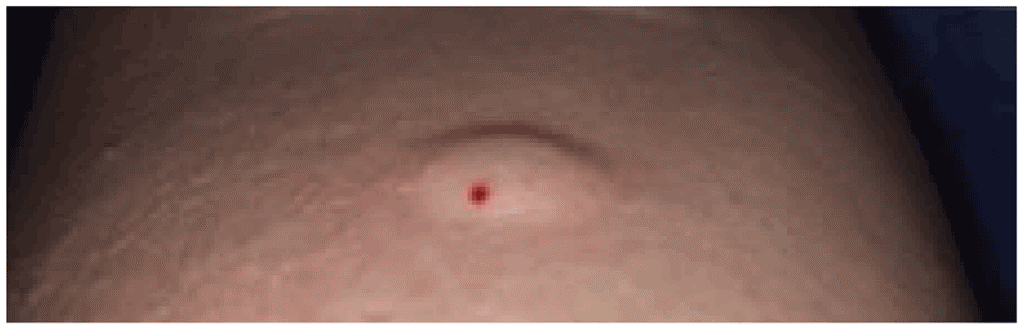
A small induration should be seen after the injection. © Richard Menzies
Step 9. Take photographs (for mHealth – if applicable)
If an mHealth QC programme is in place, then the provider should take several photographs of the injection site. The syringe should be placed with gradation markings visible, 2 cm away from the injection site (towards the elbow). Once the patient has left, the best three photographs should be sent to the supervisor.
Step 10. Provide post-TST care
The patient should remain seated in the clinic area for 10–15 minutes. This is for surveillance in case of allergy or anaphylaxis, which is rare (1 per million) (25), or vasovagal fainting, which is much more common than anaphylaxis and can result in injury from an unprotected fall. If an individual feels faint during or after injection, then ensure that the person is protected from falling and immediately record vital signs, particularly blood pressure and heart rate. If the heart rate is rapid and the blood pressure low, and particularly if there are any other signs of anaphylaxis, provide care for a potential anaphylactic reaction. If the heart rate is low (<60 beats per minute), then this is much more likely to be a simple vasovagal faint. Position the patient with their head down and use other manoeuvres effective for vasovagal reaction.
At the time of discharge, educate the patient about care of the injection site; in particular, not to scrub vigorously when washing or to scratch if there is significant itching. If there is any blistering or pain, advise the patient that they can use cold compresses and take nonsteroidal anti-inflammatories for relief.
Step 11. Arrange for the result to be read
Remind the patient of the need to return 48–72 hours later (24–72 hours for C-TST) for the result to be read and provide them with an appointment (with date and time) to come for the reading. Flexibility is important to accommodate patient schedules. Ideally, the reading should be scheduled after 48 hours, so that if the patient is unable to come, the reading can be rescheduled for the next day and still fall within the 72-hour maximum window for reading. The person tested should be aware that the reading is not valid if more than 72 hours have elapsed since administration.
Step 12. Record the result and document details
In the patient’s medical record or on specific forms or registries, as appropriate, it is important to record the following details regarding the test administration:
- name of person administering the TST or TBST;
- date and time of administration;
- product used and lot number;
- date on which the product vial was opened (if vials are used over multiple days, based on manufacturer instructions);
- site of injection;
- whether a bleb was seen, and any bleeding or leakage of test fluid;
- any adverse event – if the patient had hypotension or loss of consciousness, it is crucial to document whether this was due to vasovagal reaction or anaphylaxis; and
- date and time of appointment for reading.
A2 – Reading of TST or TBST result
Step 1. Seeing the patient
Patients should be seen as quickly as possible after they arrive for the reading of their TST or TBST. Reading of the test takes less than 3 minutes on average (7). If a person must wait a long time simply for the result to be read, this may discourage other contacts in the same household from coming forward for testing or reading, and may discourage the same person from having a re-test, if needed.
Step 2. Re-check symptoms
If the person had non-TB respiratory symptoms at the time the test was administered, then these symptoms should be re-checked at the time of reading. If the symptoms are less severe or have resolved, then this individual can be considered to have “no symptoms” in the algorithm. However, if symptoms have not improved – and particularly if the symptoms have worsened or are suggestive of TB (e.g. fever or night sweats) – the individual should be considered to have symptoms for investigation. In such cases, the result of the TST or TBST should be read and recorded, but the person should be referred promptly for medical evaluation, including chest X-ray (CXR) – if not already done – and other testing as appropriate, regardless of the result of the test for TB infection.
Step 3. Place and position of patient for reading
Reading should be done in a private room (wherever possible), out of view of all other individuals (although family members can attend, as appropriate). For optimal measurement, the patient should be seated with the arm supported.
Step 4. Inspection of injection site
The site of the injection for the TST or TBST should be carefully inspected. If there is blistering, skin breakdown or lymphadenitis these should be recorded because they are considered to be strong positive reactions for all TB infection skin tests.
Erythema or redness is an indicator of potential induration (if erythema is present, then induration may be present). However, erythema does not need to be measured for TST (3), or for Cy-Tb (6), and only needs to be measured for Diaskintest if there is no induration (5). For C-TST, erythema should be demarcated and measured (4).
Induration or firm swelling can be detected visually or by palpation.
If there is no redness and no visible or palpable induration, then the test is negative and the result is marked as “0 mm”. Likewise, if there is erythema but no obvious swelling, and on light palpation there is no evidence of induration, then the induration can be considered negative and “0 mm” can be recorded. If any swelling or induration can be palpated, then this should be demarcated (see Step 5) and measured.
If an mHealth QC programme is in place, then photographs of the injection site should be taken.
Step 5. Demarcation of induration
For TST, Cy-Tb and Diaskintest, the induration is measured (3, 5, 6). For C-TST, both induration and erythema are measured, and the largest of the two is used for clinical management (4).
To demarcate the edges of the induration, the ballpoint pen method can be used (8). With this method, a ballpoint pen tip is pushed gently against the skin at a 45-degree angle towards the site of the injection. If there is a firm and distinct induration, the ballpoint pen tip will consistently stop at the margins. This procedure is repeated several times from different directions around the injection site. If there is no visible or palpable induration, it is not necessary to use a ballpoint pen; the result can be marked as “0 mm”.
Large reactions can be painful, and it is not necessary to insist on demarcating and measuring large painful indurations. These can be simply marked as “greater than 15 mm” or “greater than 20 mm” and any blistering or skin necrosis noted.
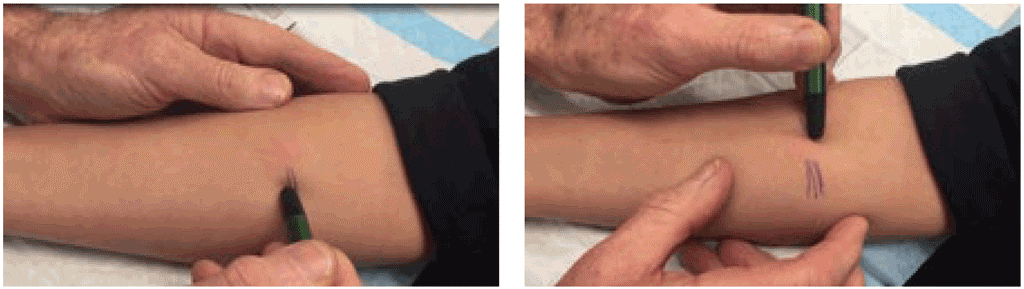
Demarcate the edges of the induration with a ballpoint pen several times from different directions. © Richard Menzies
Step 6. Measurement
Once the edges of the induration (or, in the case of C-TST, the erythema) have been demarcated, then the diameter of the induration should be measured. For Cy-Tb and TST, the transverse diameter is measured and recorded in millimetres (3, 6). For the C-TST, the transverse and longitudinal diameters of erythema and induration are measured, and the average of each is recorded (4). The largest of these two will be taken as the clinically relevant information. For the Diaskintest, the largest diameter of the induration in any dimension is recorded (5).
Measurement of the size of reaction is often subject to rounding error, because readers tend to group readings at 5, 10 and 15 mm. To avoid this error, it is a good practice to use machinist calipers or tailor calipers.
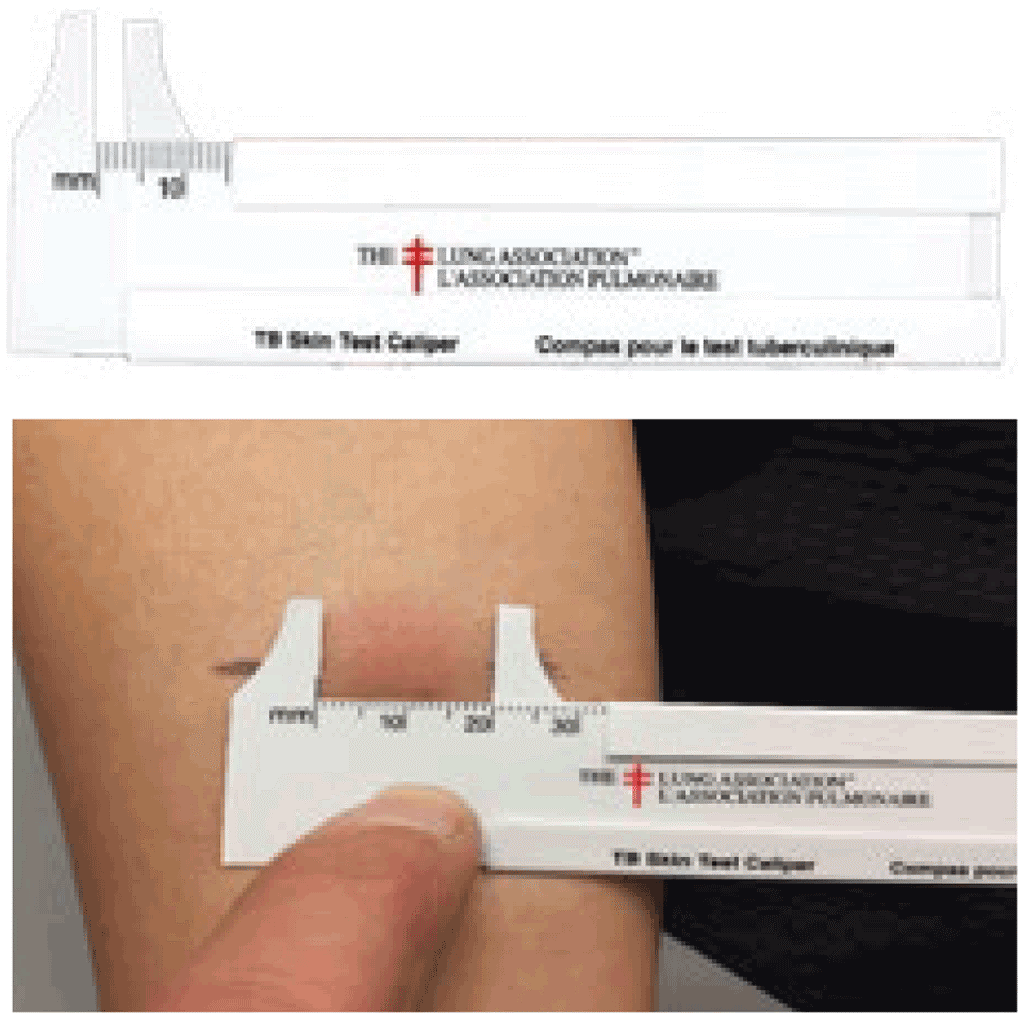
Measure the induration between the demarcations. © Richard Menzies
Step 7. Post-TST care
If the patient has blistering or skin breakdown, then it is important to prevent secondary infection. The area should be carefully cleaned and covered with a dry dressing. Patients should be instructed not to scratch the area.
Topical steroids should not be used, because these were shown to be ineffective in placebocontrolled trials (9). Subcutaneous injection of steroids under the induration may be more effective, but conservative management with cold compresses and dry dressings to cover the site is usually sufficient and prescription of oral analgesics (aspirin or acetaminophen/ paracetamol).
Step 8. Management of results
If the test is negative (as per Chapter 3 of the main text) and the patient is asymptomatic (or if symptoms are resolving), then the patient can be discharged. In some settings, close contacts will have a second TB infection test 8 weeks after the end of exposure if an initial test is negative – particularly in those with very recent exposure or concomitant viral infection. In this case, an appointment should be made for administration of the second test.
If the test is positive the person should be referred immediately for medical evaluation and CXR. In a well-organized person-centred care cascade, medical evaluation (including CXR) is available at the same site and on the same day. This minimizes delays between the first identification of a contact and the initiation of appropriate therapy for TB disease or infection.
Step 9. Recording and registration
The TST or TBST reading should be recorded in the patient’s medical record or registries (or both); additional information may be recorded, depending on the setting and programme organization.
The following should be recorded:
- date and time of reading;
- product used and lot number;
- size of the induration in millimetres (transverse, maximal or average diameter, depending on the TST or TBST);
- for C-TST, the average diameter of erythema should also be recorded, and for Diaskintest, erythema (but only if there is no induration); for the TST and Cy-Tb, erythema does not need to be recorded;
- presence of blistering, skin necrosis, lymphangitis or lymphadenopathy; and
- for those with positive tests – disposition: date and time of follow-up for medical evaluation and CXR; for those with negative tests, date and time of follow-up appointment, if appropriate.
Certain articles provide useful general information on skin tests for TB infection (10, 11).
References for Annex 4
- Moayedi-Nia S, Barss L, Oxlade O, Valiquette C, Ly MX, Campbell JR et al. The mTST – an mHealth approach for training and quality assurance of tuberculin skin test administration and reading. PLoS One. 2019;14:e0215240 (https://doi.org/10.1371/journal.pone.0215240).
- Menzies D, Doherty T. Diagnosis of latent tuberculosis infection. In: Raviglione M (ed), Reichman and Hershfield’s tuberculosis, a comprehensive international approach. New York: Informa Healthcare USA; 2006:215–63 (https://www.taylorfrancis.com/chapters/edit/10.3109/9780203908464–15/diagnosis-latent-tuberculosis-infection-dick-menzies-mark-doherty).
- The WHO standard tuberculin test. Geneva: World Health Organization; 1963 (https://apps.who.int/iris/handle/10665/112241).
- C-TST package insert. China: Anhui Zhifei Longcom; 2022.
- Diaskintest package insert. Russian Federation: Generium; 2022 (https://www.generium.ru/en/products/diaskintest/).
- Cy-Tb package insert. India: Serum Institute; 2022.
- Alsdurf H, Hill P, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1269 (https://doi.org/10.1016/S1473-3099(16)30216-X).
- Sokal J. Measurement of delayed skin-test responses. New Eng J Med. 1975;293:501–2 (https://doi.org/10.1056/NEJM197509042931013).
- Hanson M, Comstock G. Efficacy of hydrocortisone ointment in the treatment of local reactions to tuberculin skin tests. Am Rev Respir Dis. 1968;97:472–3 (https://pubmed.ncbi.nlm.nih.gov/5638501/).
- Lewinsohn D, Leonard M, Lobue P. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111–5 (https://pubmed.ncbi.nlm.nih.gov/28052967/).
- Campbell J, Pease C, Daley P, Pai M, Menzies D. Diagnosis of tuberculosis infection. In: Menzies D (ed), Canadian tuberculosis standards – 8th edition. Ottawa: Canadian Thoracic Society; 2022:49–65 (https://doi.org/10.1080/24745332.2022.2036503).

 Reacción
Reacción