Перекрёстные ссылки книги для ПЛАТФОРМА ВОЗ ПО ОБМЕНУ ЗНАНИЯМИ О ТБ
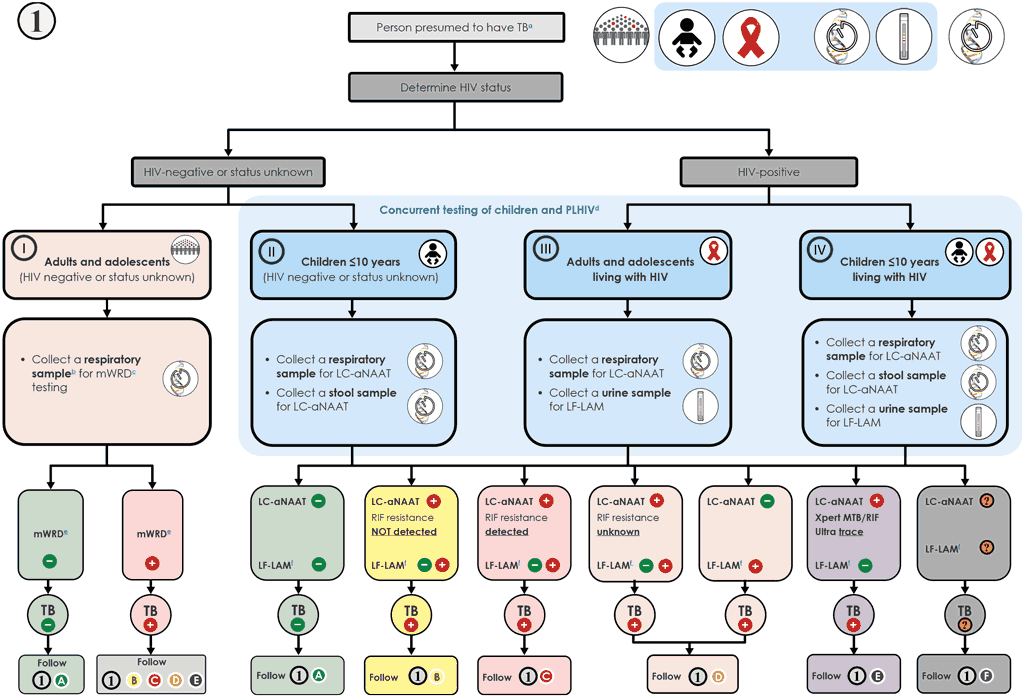
Algorithm 1 is the starting point for the diagnostic pathway, and in this context the recommended classes of WRDs include both molecular tests (LC-mNAAT, LC-aNAAT and MC-aNAAT) and a biomarker-based test (LF-LAM) (Fig. 6.2). Member States can choose the mWRD that best fits their circumstances, with the ultimate objective being to serve patient needs and ensure universal access to diagnostic and RIF-resistance testing. The mWRD classes provide different types of results:
- TB diagnosis only, using an LC-mNAAT;
- TB diagnosis and detection of RIF resistance (simultaneously or as a two-step process) using an LC-aNAAT; or
- TB diagnosis and detection of RIF and INH resistance (simultaneously or as a two-step process) using an MC-aNAAT.
Where an LC-mNAAT is used, follow-on testing for RIF resistance may be needed.
LF-LAM provides a result for TB diagnosis only (without RIF resistance). Recommendations for the urine LF-LAM have been expanded to apply to all individuals infected with HIV, regardless of setting and CD4 count; also, the test has been integrated with LC-aNAATs in the recently recommended concurrent testing strategy for adults, adolescents and children living with HIV. The recommendations on concurrent testing do not include the use of LC-mNAATs or MC-aNAATs.
The test options for Algorithm 1 vary in complexity. LF-LAM is the only POC test for the initial detection of TB that does not require special skills for testing or laboratory infrastructure. The LC-aNAATs require basic pipetting skills and are easy to decentralize to basic laboratories; however, they have limited throughput with the commonly used instruments. In contrast, some MC-aNAATs require more hands-on time and have large infrastructure requirements; most of them provide high throughput and are best suited to established laboratories with reliable sample referral networks. In practice, test needs and associated choices are likely to vary, depending on the setting within a country or province. Consideration should be given towards hybrid models that use a combination of tests from different manufacturers; this has the added advantage of providing a safety mechanism in the event of an expected problem with a supplier.
It is feasible to implement Algorithm 1 when the mWRD testing can be conducted onsite or can be accessed through a reliable referral system with short turnaround times.
Fig. 6.2. Algorithm 1: mWRD as the initial diagnostic test for TB
Footnotes are interactive
CSF: cerebrospinal fluid; CXR: chest X-ray; DNA: deoxyribonucleic acid; DST: drug susceptibility testing; HIV: human immunodeficiency virus; Hr-TB: isoniazid-resistant, rifampicin-susceptible TB; LAMP: loop-mediated isothermal amplification; LC-aNAAT: low-complexity automated NAAT; LC-mNAAT: low-complexity manual NAAT; LF-LAM: lateral flow urine lipoarabinomannan assay; LPA: line probe assay; MC-aNAAT: moderate-complexity automated NAAT; MC-mNAAT: moderate-complexity manual NAAT; MDR-TB: multidrug-resistant TB; MTB: Mycobacterium tuberculosis; MTBC: Mycobacterium tuberculosis complex; mWRD: molecular WHO-recommended rapid diagnostic test; NAAT: nucleic acid amplification test; NGS: next-generation sequencing; PLHIV: people living with HIV; RR-TB: rifampicin-resistant TB; TB: tuberculosis; TPT: TB preventive treatment; WHO: World Health Organization.
Drugs and regimens: INH: isoniazid; HREZ: isoniazid (H), rifampicin (R), ethambutol (E) and pyrazinamide (Z); REZ: rifampicin (R), ethambutol (E) and pyrazinamide (Z); RIF: rifampicin.
a Persons presumed to have TB include those with signs and symptoms of TB and those who screen positive for TB. They include adults and children with signs or symptoms suggestive of TB, with a CXR showing abnormalities suggestive of TB, a positive mWRD used as a screening tool or positive C-reactive protein test (>5 mg/L) in PLHIV. A person with a positive mWRD used as a screening tool and a low pretest probability should be clinically assessed and, if deemed to be a person presumed to have TB, should have a repeat mWRD performed and follow Algorithm 1. If the pretest probability is high and the clinical picture is consistent with TB disease, then this test could be considered diagnostic and the person should be managed based on the result of the test and, if relevant, should continue on to Algorithm 2 or 3. This algorithm may also be followed for the diagnosis of extrapulmonary TB using CSF, lymph node and other tissue specimens. However, mWRDs that are recommended for use in the diagnosis of extrapulmonary TB investigations are currently limited to Xpert Ultra.
b Programmes may consider collecting two specimens upfront. The first specimen should be promptly tested using the mWRD. The second specimen may be used for the additional testing described in this algorithm. For individuals being evaluated for pulmonary TB, sputum is the preferred specimen. Tissue biopsy samples are difficult or impossible to obtain repeatedly; therefore, they should be tested with as many methods as possible (e.g. mWRD, culture, DST or histology).
c mWRD includes LC-aNAAT, LC-mNAAT and MC-aNAAT for the general population.
d The concurrent testing strategy was only assessed for LC-aNAAT in combination with LF-LAM owing to lack of evidence for LC-mNAAT and MC-aNAAT. A laboratory equipped only with LC-mNAAT or MC-aNAAT can also use these tools to diagnosis both of PLHIV and children because both classes are recommended for initial diagnosis of TB of these groups. Additional testing may be required to detect RIF resistance if LC-mNAAT was used. This strategy is not covered by the concurrent testing recommendations but is a way to increase sensitivity in the diagnostic cascade without unnecessary delays. An initial positive result on any of these tests, LC-mNAAT, MC-aNAAT or LF-LAM should lead to treatment initiation.
e Only the result from the single mWRD is available for this group.
f LF-LAM should not be used on people who are HIV-negative.
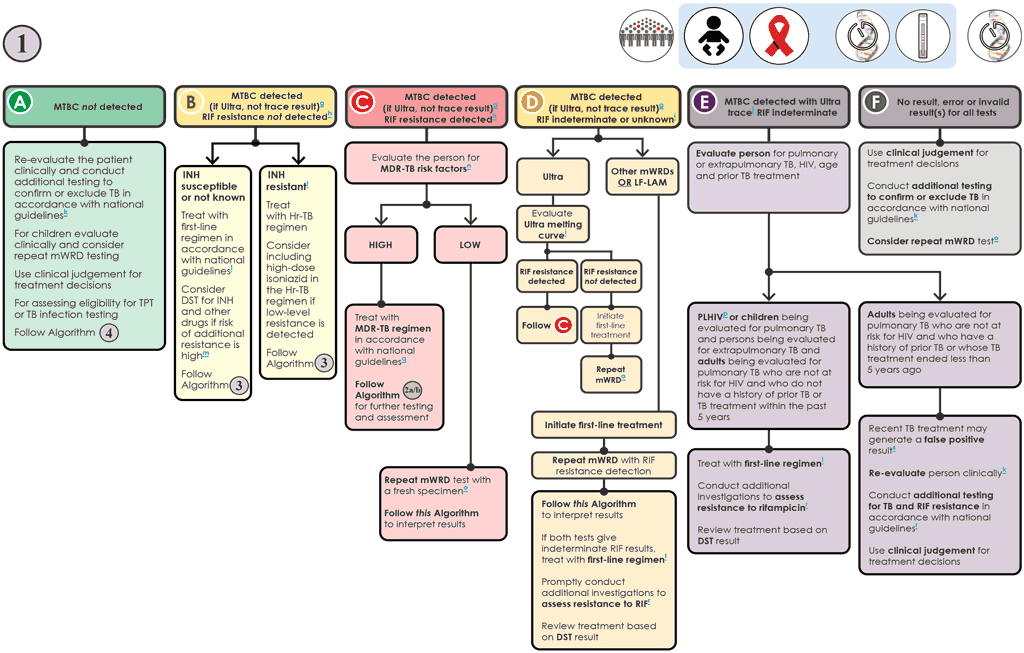
g “MTBC detected (if Ultra, not trace results)” includes MTBC detected as high, medium, low or very low. These categories apply to the Xpert Ultra tests. Results of the Truenat MTB and MTB Plus tests, MC-aNAAT and the TB LAMP test also fall into the category of “MTBC detected (if Ultra, not trace results)”. The MC-aNAAT provides additional resistance detection for INH and leads to additional considerations in pathway B.
h Determination of RIF resistance occurs simultaneously in the Xpert Ultra test and the MC-aNAAT. A reflex test is needed to determine RIF resistance for Truenat testing, using the same DNA isolated for the Truenat MTB test. The TB-LAMP test requires a fresh specimen to be collected and a molecular or phenotypic DST to be conducted. In the case of MC-aNAAT, INH resistance detection also occurs simultaneously with RIF detection.
i The interpretation and follow-up testing for MTBC detected and RIF indeterminate or unknown for the Xpert Ultra test differs from the interpretation of results for other mWRDs. MTBC detected with RIF indeterminate results obtained with the Xpert Ultra test (especially those with high and medium semiquantitative results) may be due to large deletions or multiple mutations that confer RIF resistance. Analysis of the Xpert Ultra melt curves can detect such resistance-conferring mutations. In some cases, culture and DST, sequencing or alternative mWRD will be needed to confirm or exclude RIF resistance. Indeterminate results for the other mWRDs are usually related to very low numbers of bacilli in the sample. When using an mWRD without detection of RIF resistance (e.g. TB-LAMP), further testing for RIF resistance is required preferably using an mWRD able to detect resistance.
j “MTBC detected trace” applies only to the Xpert Ultra test.
k Further investigations for TB may include CXR, additional clinical assessments, repeat mWRD testing, culture or clinical response following treatment with broad-spectrum antimicrobial agents.
l People should be initiated on a first-line regimen according to national guidelines, unless the person is at very high risk of having MDR-TB. Such people should be further investigated and initiated on an MDR-TB regimen if pertinent. In situations where INH results are available (e.g. MC-aNAAT) and INH resistance has not been detected, the probability of having MDR-TB would be lower.
m A sample may be sent for molecular or phenotypic DST if there is a high prevalence of INH or other drug resistance and RIF susceptible (i.e. INH monoresistance or polyresistance) in this setting. Where a result for INH resistance is “not detected” (e.g. MC-aNAAT), and the pretest probability for Hr-TB is high, phenotypic DST for INH should be performed.
n People at high risk for MDR-TB include people who were treated previously, including those who had been lost to follow-up, relapsed or experienced treatment failure; non-converters (smear positive at end of intensive phase); MDR-TB contacts; and any other groups at risk for MDR-TB identified in the country.
o The mWRD with RIF testing should be performed at the same testing site with a fresh specimen, and the result of the second test should be interpreted as shown in this algorithm. The RIF result from the second test is the result that should be used for clinical decisions.
p PLHIV include those who are HIV-positive or whose HIV status is unknown, but who present with strong clinical evidence of HIV infection, reside in settings where there is a high prevalence of HIV or are members of a group at risk for HIV. For all those with unknown HIV status, HIV testing should be performed according to national guidelines.
q People should be promptly initiated on an MDR-TB regimen in accordance with national guidelines. Algorithm 2 should be followed for additional testing for any person with RR-TB.
r Phenotypic (culture and DST) and molecular (e.g. alternate mWRDs, LPAs and targeted NGS) methods are available for evaluating drug resistance. Rapid molecular methods are preferred.
s In people with a prior history of TB within the past 5 years or whose TB treatment was completed less than 5 years ago, Xpert Ultra trace results (and occasionally “MTBC detected low or very low”) may be false positive, not because of active TB but because of the presence of non-viable bacilli. Clinical decisions must be made based on all available information and clinical judgement.
t People diagnosed using an MC-aNAAT and whose result is RIF resistance not detected, and INH resistance detected should be treated for Hr-TB with REZ and levofloxacin. For practical purposes, HREZ fixed-dose combination tablets may be used instead of REZ. Consider including high-dose INH in the Hr-TB regimen if low-level resistance is detected (inhA mutation only). Follow Algorithm 3.
6.3.1 Decision pathway for Algorithm 1 – WRD as the initial diagnostic test for TB
General considerations
WHO recommends the use of an mWRD from one of the LC-mNAAT, LC-aNAAT and MC-aNAAT classes as an initial diagnostic test for TB (rather than microscopy or culture) for all adult and adolescent HIV-negative individuals with signs and symptoms of TB. WHO also recommends the concurrent use of urine LF-LAM and an LC-aNAAT to diagnose TB among adults, adolescents and children living with HIV (see Section 3.4). Details on the different tests included in the different classes are given in Section 2. The target populations include:
- newly presenting individuals with symptoms of TB (cough of any duration, fever, haemoptysis, night sweats and weight loss);
- individuals who have screened positive for TB by an alternative method (e.g. CXR or C-reactive protein) and require confirmation; and
- individuals who are on treatment or have been previously treated, or people being evaluated for possible RR-TB or Hr-TB (e.g. non-converters at the end of the intensive phase of treatment despite treatment adherence) or for a new or continuing episode of TB (e.g. relapse cases or people treated previously, including those who had been lost to follow-up).
TB programmes should transition from using microscopy as the initial diagnostic test to using mWRDs. The mWRDs show a higher sensitivity for the diagnosis of TB; also, they can simultaneously detect resistance to RIF and, in cases of MC-aNAAT use, resistance to INH.
For monitoring treatment, mWRDs are not recommended, because the tests do not discriminate between live and dead bacilli and thus may generate false positive results. Instead, microscopy and culture should be used for monitoring treatment, in accordance with national guidelines and WHO recommendations. More information on the need to develop more rapid and sensitive tools for TB treatment monitoring can be found in the 2023 WHO target product profiles for tests for tuberculosis treatment monitoring and optimization (82).
Algorithm 1 describes the collection of one or more quality initial specimens to be used for mWRD testing and the collection of additional specimens as needed (information on which specimens can be used with which WRD is given in Section 2 above and in individual WHO policy recommendations):
- for adults and adolescents, specimens that may be used are induced or expectorated sputum (preferred), tracheal aspirate or bronchoalveolar lavage; in addition, urine should be used for LF-LAM testing among those who are HIV-positive;
- for children, additional specimens include gastric aspirates, nasopharyngeal aspirate and stool; in addition, urine may be used for LF-LAM testing among those who are HIV-positive; and
- for people being evaluated for extrapulmonary TB, specimens that may be used are cerebrospinal fluid, lymph node tissue aspirate, pleural tissue, pleural fluid, synovial fluid, peritoneal fluid or pericardial fluid.
For LF-LAM, midstream urine should be collected in a sterile, standard urine specimen cup at a facility with private urine collection and handwashing areas. Whenever feasible, fresh samples should be tested, ideally immediately after collection. If immediate testing is not possible, urine should be stored according to the LF-LAM manufacturer instructions for use (24). LF-LAM testing is appropriate for all persons living with HIV being evaluated for pulmonary or extrapulmonary TB, regardless of the overall prevalence of HIV in the setting. The LF-LAM test result (<30 minutes) is likely to be available before the mWRD result and, if positive, is considered as bacteriological confirmation of TB.While LF-LAM does not differentiate between various species of the genus Mycobacterium, in areas with a high prevalence of TB, the LAM antigen detected in a clinical sample is likely to be attributed to MTBC.
For operational issues, programmes may consider routinely collecting two specimens (e.g. spot and morning sputum samples, or two spot specimens) from each HIV-negative person or child, instead of only collecting a second specimen when additional testing is needed. If two specimens are collected, the first should be tested promptly using the first available mWRD. The second specimen may be used for the additional testing described in the algorithm (e.g. repeat mWRD testing for failed tests or follow-on resistance testing, or for smear microscopy or culture as a baseline for treatment monitoring).
If only one specimen can be collected (e.g. if it is difficult or impossible to obtain tissue biopsy samples), the TB diagnostic algorithm should be modified to prioritize testing with the mWRD. If additional TB testing is warranted, one option is to consider using any portion of the sample remaining after the mWRD for other tests (e.g. culture, histology, LPA and DST). Alternatively, the sample could be processed for culture in an appropriately equipped laboratory, and the same sediment could be used for the mWRD, culture and other tests. Clinical decisions should be made based on clinical judgement and the results of available laboratory tests.
With respect to the detection of MTBC, mWRD results are typically reported as “MTBC not detected”, “MTBC detected”, “no result”, “error” or “invalid”. Within the “MTBC detected” result group, some mWRDs provide semiquantitative results (high, medium, low or very low). The Xpert Ultra test has an additional semiquantitative category called “trace”.
- When Xpert Ultra “trace” is used for people living with HIV and children who are being evaluated for pulmonary TB, and for individuals being evaluated for extrapulmonary TB, the “MTBC detected trace” result is considered as bacteriological confirmation of TB.
- In adults who are HIV-negative and symptomatic, with a recent history of TB treatment (i.e. completed <5 years ago), Xpert Ultra “trace” results (and occasionally other mWRD “MTBC detected very low”) may be positive not because of active TB but because of the presence of non-viable bacilli. Clinical decisions must be made on all available information and clinical judgement.
With respect to the detection of RIF and INH resistance, mWRDs report the results as RIF or INH “resistance detected”, “not detected” or “indeterminate”. For the assays for which resistance detection relies on the absence of binding of wild-type reporter probes to amplicons (e.g. Truenat MTB-RIF Dx) it may be advisable to state that resistance is inferred rather than detected.
When two mWRDs are performed (in the context of the concurrent testing strategy), and the results of the two tests are discordant for RIF status, possible errors should be investigated (e.g. wrongly labelled samples or machine error) and the resistance profile of contacts reviewed. If the investigation in inconclusive, the resistant results should be used; alternatively, the test could be repeated with the resistant sample type before a DR-TB regimen is prescribed.
The use of an mWRD to detect resistance to RIF or INH (or both) may not eliminate the need for follow-up DST.
Decision pathway
The results of the test or tests should be used to guide next steps and inform treatment decisions. A description of pathway selection is given below, followed by the considerations and actions for each of the WRD pathways.
- If all tests are negative, Pathway 1A applies.
- If any test is positive, the test results guide selection of Pathways B to E:
- Pathway 1B : MTBC detected, and RIF susceptibility confirmed;⁶
- Pathway 1C : MTBC detected and RIF resistance detected;
- Pathway 1D : MTBC detected (if Ultra, not trace result) and RIF resistance unknown or indeterminate; and
- Pathway 1E : MTBC detected with trace result, RIF indeterminate.
- If all tests yield no results, invalid results or errors, Pathway 1 F applies.
When conducting concurrent testing of multiple samples and tests for people living with HIV and children, Pathway 1A applies if all tests are negative, whereas Pathways 1B to 1E apply if any test is positive. Pathway 1B applies when all resistance tests indicate RIF susceptibility, whereas Pathway 1C applies if any test indicates RIF resistance. For patients who test positive for TB with LF-LAM or LC-mNAAT but without the initial availability of a RIF-resistance result and without access to LC-aNAAT, an additional sample should be collected and transported for testing with an LC-aNAAT offsite. Pathway 1D may be followed until the RIF-resistance result is available.
For figure notes, please see page 112.
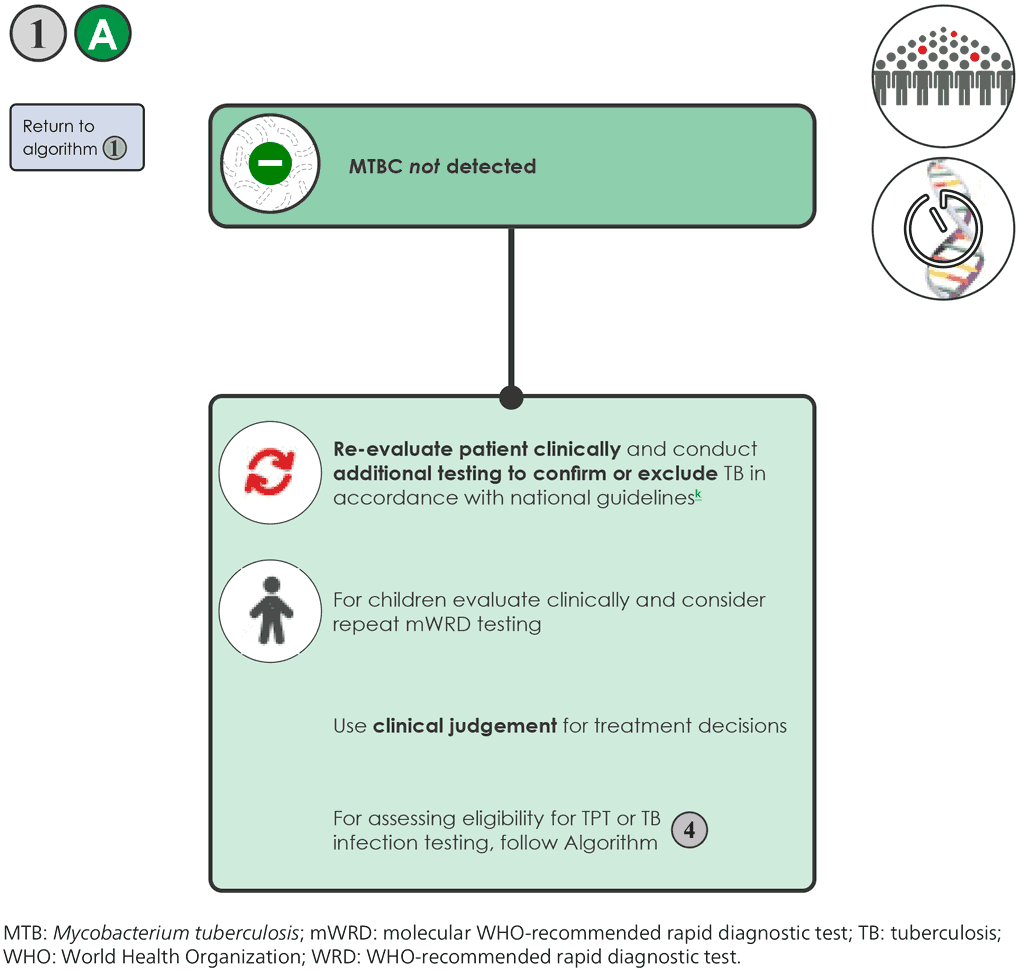
- If the mWRD result is “MTB not detected” 1A , the person should be re-evaluated, and additional testing undertaken in accordance with national guidelines.
- For the concurrent testing, all tests included in the strategy must be negative to follow this pathway, including LF-LAM tests among persons living with HIV.
- Further investigations for TB may include CXR, additional clinical assessments, additional mWRD testing, or culture and clinical response following treatment with broad-spectrum antimicrobial agents (FQs should not be used).
- For children, a careful clinical assessment is necessary to rule out TB, as detailed in the WHO operational handbook on tuberculosis: module 5: management of tuberculosis in children and adolescents (58).
- For persons living with HIV, treatment for Pneumocystis pneumonia should be considered. If there is clinical worsening or no improvement after 3–5 days of treatment, further investigations for TB and other diseases may be re-initiated and, if the person is seriously ill with danger signs, presumptive TB treatment should be initiated.
- For persons living with HIV with advanced HIV disease, the AHD package of care should be applied irrespective of the negative test results. All children living with HIV aged under 5 years are considered as having AHD.
- As always, clinical judgement should be used for treatment decisions, including considering the possibility of clinically defined TB (i.e. TB without bacteriological confirmation).
For figure notes, please see page 112.
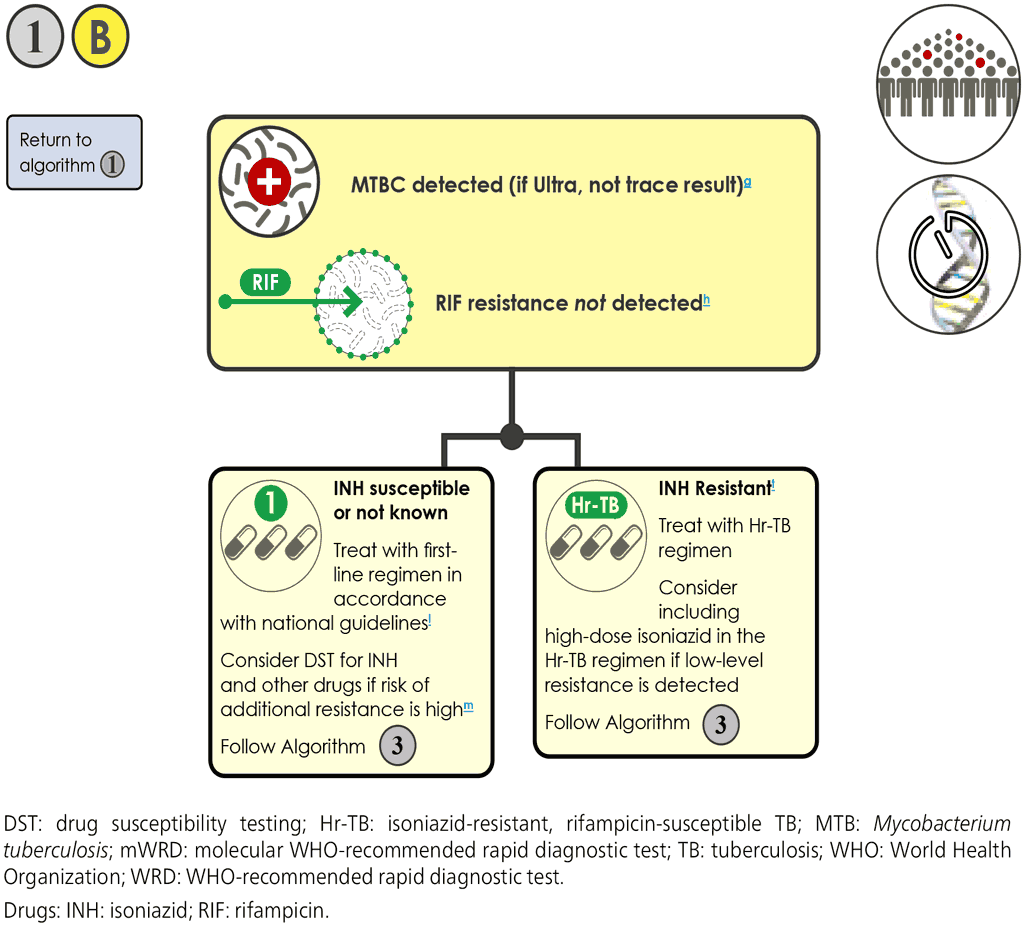
- If the mWRD result is “MTBC detected, RIF resistance not detected” and “INH resistance not detected” or INH results are unknown: 1B.
- For children, this applies when either or all of the LC-aNAATs are “MTBC detected, RIF resistance not detected”. If one LC-aNAAT shows “MTBC detected, RIF resistance not detected” and one LC-aNAAT shows “MTBC detected, RIF indeterminate”, the test should be repeated on the specimen type that initially tested as RIF indeterminate and the final valid result should be used for decision-making.
- For people living with HIV, this applies regardless of the LF-LAM result.
- The person should be initiated on an appropriate regimen using first-line TB drugs in accordance with national guidelines.
- Additional DST should be requested in the following cases:
- Molecular or phenotypic DST for INH is indicated:
- if the person has been treated previously with INH or is a contact of a person known to have Hr-TB; or
- if there is high prevalence of INH resistance that is not associated with RIF resistance (i.e. Hr-TB or polyresistance, not MDR-TB) in this setting;
If the INH resistance is “not detected” by an MC-aNAAT and the person has a high risk of Hr-TB, phenotypic DST for INH should be performed because 6–14% of INH resistance can be missed by MC-aNAATs.
- Molecular or phenotypic DST for RIF resistance may be requested if the person is at risk of having RR-TB despite the initial mWRD result showing “resistance not detected”. Sometimes, these anomalous results may be due to sample handling errors and a repeat test may resolve the issue. False RIF-susceptible Ultra results can occur but are uncommon (1–5% of RIF-resistant TB cases tested) and the level of such results depends on the epidemiological settings. In contrast, phenotypic DST for RIF, especially using liquid culture, is associated with a higher proportion of false susceptible results (83). The updated critical concentration for RIF should be used to reduce, but not eliminate, this issue. Sequencing should be performed when available, and should cover codons 170–491 of the rpoB gene (including those outside of the rifampicin resistance determining region, or RRDR).
- Molecular or phenotypic DST for INH is indicated:
- If additional molecular or phenotypic testing is performed:
- Testing done in different laboratories should be done in parallel – it is important to not wait for the results of one test before initiating another test.
- Molecular and phenotypic DST may be performed using the specimen (direct DST) or using a culture isolate (indirect DST). Direct DST is preferred for molecular testing, whereas indirect DST may be preferred for phenotypic DST because of technical issues related to producing an appropriate inoculum and loss to contamination.
- An mWRD is preferred. Mutation interpretation can also be found in the WHO catalogue of mutations (23). Targeted NGS is an accurate method for DST that can produce results faster than culture-based DST. Targeted NGS shows a sensitivity of almost 90% or above for detection of resistance to INH, LFX, MXF, PZA, AMK, ETB and STR, and about 70% for BDQ, LZD and CFZ for RIF-resistant samples.
- Culture-based phenotypic DST for INH and RIF often require 3–8 weeks to produce results, but may be useful for evaluating people with a susceptible molecular result, particularly in populations with a high pretest probability for resistance to INH. If the mWRD result is “MTBC detected, RIF resistance not detected and INH resistance detected” (currently only applicable to the MC-aNAAT):
- The person should be initiated on an appropriate Hr-TB regimen in accordance with national guidelines. The WHO recommendation for people with Hr-TB is treatment with RIF, EMB, PZA and LFX for a duration of 6 months (7).
- For individuals with Hr-TB, Algorithm 3 should be followed:
- Additional DST for RIF may be required in settings where RIF-resistant mutations outside the RRDR are common. The decision on the choice between phenotypic testing or sequencing will depend on the availability of sequencing and the type of mutation expected. In places where the rpoB I491F mutation is common, sequencing is preferred because phenotypic DST, even with the lower CC, will still miss many resistant infections; in other cases (e.g. V170F) phenotypic testing is appropriate (84).
For figure notes, please see page 112.
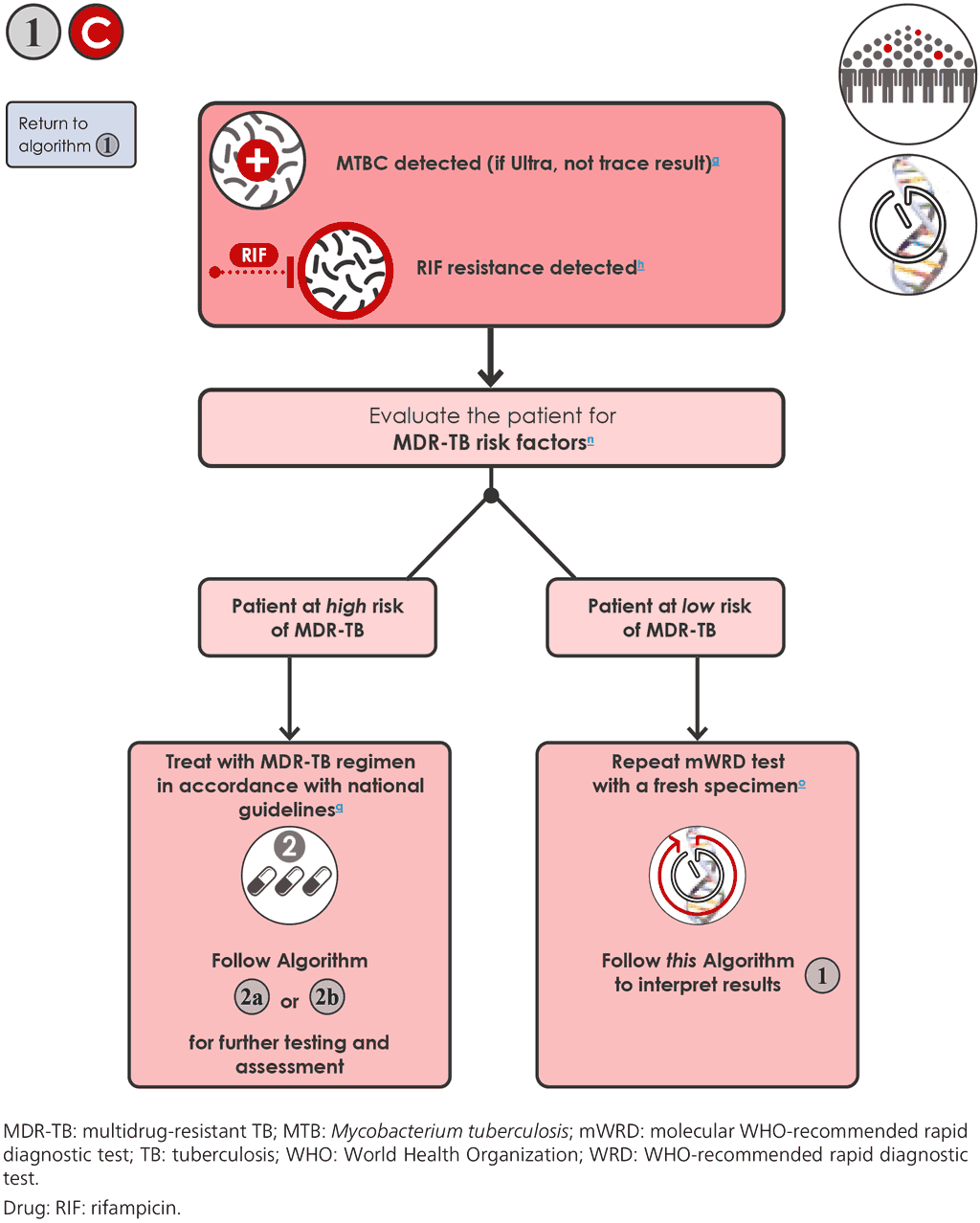
- If the mWRD result is “MTBC detected, RIF resistance detected” 1C , an MDR-TB risk assessment is needed, irrespective of the INH result. People at high risk for MDR-TB include people who have been previously treated (e.g. those who had been lost to follow-up, relapsed or experienced a failed treatment regimen); non-converters (e.g. people who were smear positive at that end of the intensive phase of treatment for drug-susceptible TB); contacts of people with MDR-TB; and any other groups at risk for MDR-TB identified in the country. In high MDR-TB 1C burden countries, every person with TB is considered to be at high risk of having MDR-TB.
- For the concurrent testing strategy, applies when at least one LC-aNAAT is “MTBC detected, RIF resistance detected”. The LF-LAM results need not be taken into consideration in this case.
- If the person is at high risk of having MDR-TB, they should be initiated on a regimen for MDR/RR-TB in accordance with national guidelines, and Algorithm 2a or 2b should be followed for additional testing.
- If the person is not at high risk of having MDR-TB, the test should be repeated using an mWRD with a second sample. To aid interpretation, the initial instrument output for the result can be reviewed when available. Probe binding delay and samples that have low bacillary loads (e.g. Xpert Ultra semiquantitative low and very low categories) have been associated with increased false resistance in some settings (85–87).
- If the second test also indicates RIF resistance, an MDR/RR-TB regimen should be initiated in accordance with national guidelines and WHO recommendations, and Algorithm 2a or 2b should be followed for additional testing.
- If the mWRD result for the second sample is “MTBC detected, RIF resistance not detected”, treatment should be initiated with a first-line regimen in accordance with national guidelines. In most situations, false positive RIF-resistant results due to technical performance of the assay are rare; however, such results may occur because of laboratory or clerical errors. It is assumed that the repeat test will be performed with more caution, that the result of the second test will be correct, and that the result of the first test may have been due to a laboratory or clerical error. Mixed infections in high-burden settings could also explain such discordance; therefore, people should be closely followed up and tested again if the response to first-line treatment is poor. If an INH resistance or susceptibility result is available, the result should be interpreted and followed up as described in Algorithm 3.
- If the mWRD result for the second sample is “MTBC detected, RIF resistance indeterminate”, the person will require further investigation. A possible mixed infection may explain such a scenario. History of prior treatment and TB contact history should be reassessed. The decision to manage a person as having Hr-TB or MDR/RR-TB will need to be based on further investigation that includes phenotypic DST to RIF and INH and, where available, DNA sequencing. A third mWRD should be performed to decide on the initial therapy; the person should be closely followed up while awaiting the final definitive results, and the appropriate algorithm should be followed.
- If an MC-aNAAT was performed and INH results are also available, this could be useful to provide certainty. The finding of INH resistance should prompt further investigation to exclude RIF resistance.
- For all people with MDR/RR-TB, additional investigations should be conducted to assess resistance to the drugs being used in the treatment regimen. Rapid detection of FQ resistance is essential in determining the regimen to be selected. The recent addition of an LC-aNAAT for the detection of FQ resistance provides a rapid and accurate peripherallevel solution that can be performed directly on specimens. The sample preparation for the Xpert MTB/XDR test is identical to the Xpert MTB/RIF Ultra test. If more than 2 mL of that reaction mix (the minimum volume to add to the Xpert cartridge) used for the Xpert Ultra test remains, this can be used directly for the Xpert MTB/XDR test if the mix has been stored at 2–8 ˚C for less than 4 hours.
- Phenotypic methods (culture and DST) and molecular methods (e.g. LC-aNAAT, SL-LPA and targeted NGS) are available to evaluate drug resistance beyond RIF and INH. Rapid molecular methods are preferred. However, for resistance detection to some of the new and repurposed drugs, targeted NGS should be used where available, otherwise phenotypic DST should be performed if it is the only available option; thus, two separate specimens may be required.
- MDR/RR-TB regimens rely on the use of BDQ with or without an FQ (a sample should be submitted for targeted NGS or molecular testing for FQ resistance plus phenotypic DST – see Algorithm 2a or 2b).
- Ideally, a specimen from each person should be submitted for DST for each of the drugs used in the regimen for which there is a reliable testing method. However, treatment initiation should not be delayed while waiting for DST results (e.g. phenotypic DST can take weeks or even months to provide results).
- Any positive culture recovered during treatment monitoring that is suggestive of treatment failure should undergo DST for the drugs used in the treatment regimen.
For figure notes, please see page 112.
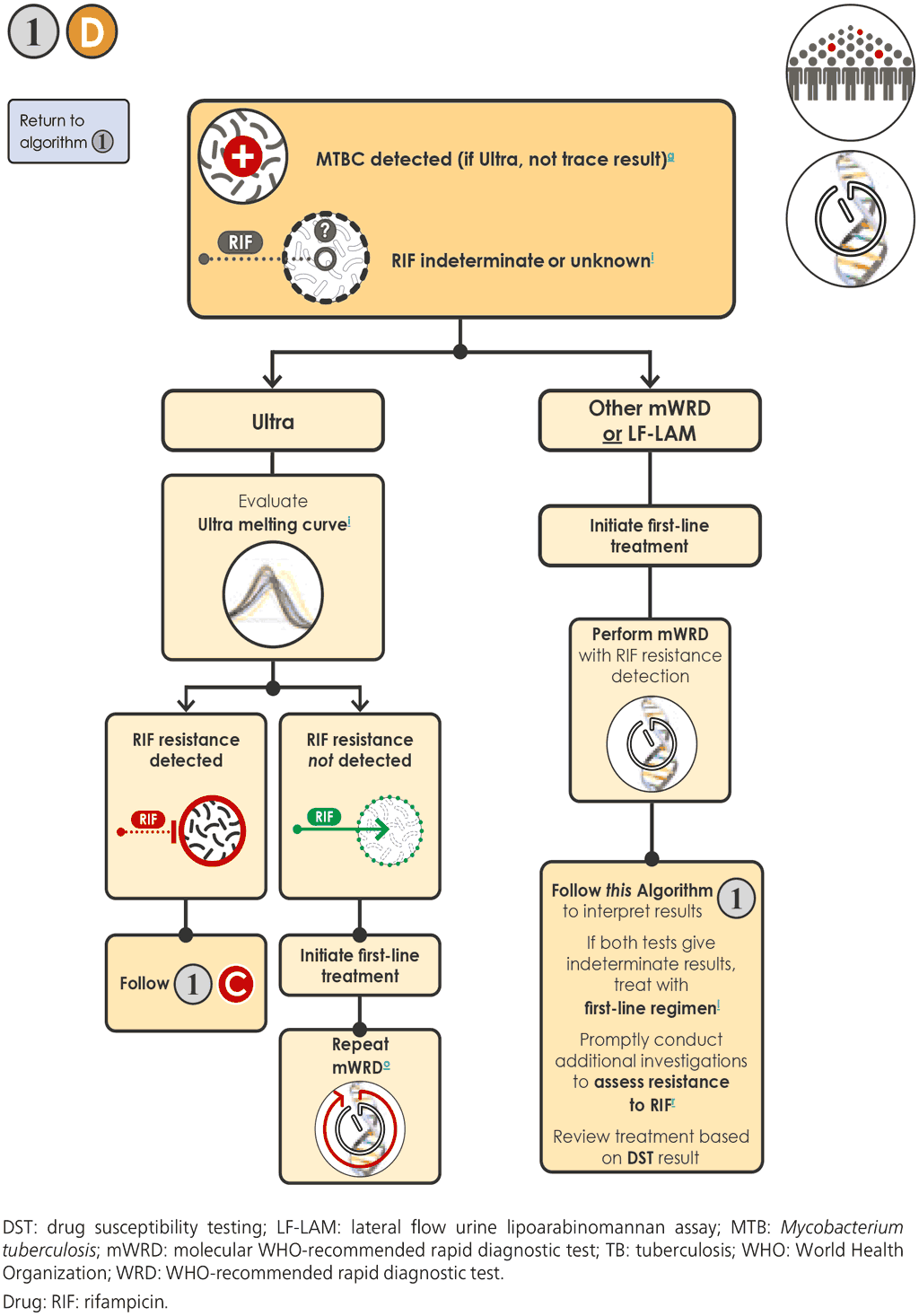
- If the mWRD gives a result of “MTBC detected, RIF indeterminate” 1D , the person will require further investigation. This also applies to concurrent testing with LC-aNAAT and LF-LAM (for people living with HIV) when only the LF-LAM is positive. The interpretation and follow-up testing for Xpert Ultra differs from that for other mWRDs. With any of the mWRDs and for LF-LAM, the initial result of “MTBC detected” (or “Positive” for LF-LAM) should be considered bacteriological confirmation of TB. The person should be initiated on an appropriate regimen using first-line TB drugs in accordance with national guidelines. If the person is at high risk of having MDR-TB, the next step is either to initiate MDR-TB treatment based on local guidelines or to initiate first-line treatment and refer to the local MDR treatment committee for a final decision. In most settings, for the purpose of making treatment decisions, a history of prior TB treatment is not sufficient to indicate that the person is at high risk of having MDR-TB.
- For most mWRDs, an “MTBC detected, RIF resistance indeterminate” result is generally caused by a paucibacillary TB load in the sample; in such cases, retesting a fresh specimen is recommended.
- If the result of the second mWRD is “MTBC detected, RIF resistance not detected”, Step 3 should be followed. If the result is “MTBC detected, RIF resistance detected”, Step 5 should be followed.
- In some cases, testing a second sample, which might also contain very few bacteria, may generate a result of “MTBC detected, RIF indeterminate” or “MTB not detected”. In these situations, additional investigations such as culture and phenotypic DST or molecular testing of the isolate or sequencing may be needed to confirm or exclude resistance to RIF, because the indeterminate result provides no information on resistance. “MTBC detected (not trace), RIF indeterminate” results obtained with the Xpert Ultra test (especially those with high or medium semiquantitative results) may be due to the presence of large deletions or multiple mutations in the RRDR, or to mutations that pose a challenge with mutation analysis software (88).
- The Xpert Ultra melt curves from “MTBC detected (non-trace), RIF indeterminate” samples should be reviewed (preferably by an advanced Xpert user or supervisor), including a review of the amplification of the target sequences and melt curve profile (88).
- Melt curves that suggest the presence of a large deletion or multiple mutations in the RRDR should be interpreted as “RIF resistance detected”. In such cases, Steps 4.b and 4.c should be followed.
- If the melt curve is not consistent with the presence of a large deletion or multiple mutations in the RRDR, the result is interpreted as “indeterminate”. In such cases, Step 5a.ii should be followed for additional testing.
- If the semiquantitative result is high or medium, FL-LPA or DNA sequencing may be useful.
- Culture and phenotypic DST, FL-LPA or DNA sequencing may be performed for follow-up testing, to confirm or exclude RIF resistance.
- For most mWRDs, an “MTBC detected, RIF resistance indeterminate” result is generally caused by a paucibacillary TB load in the sample; in such cases, retesting a fresh specimen is recommended.
For figure notes, please see page 112.
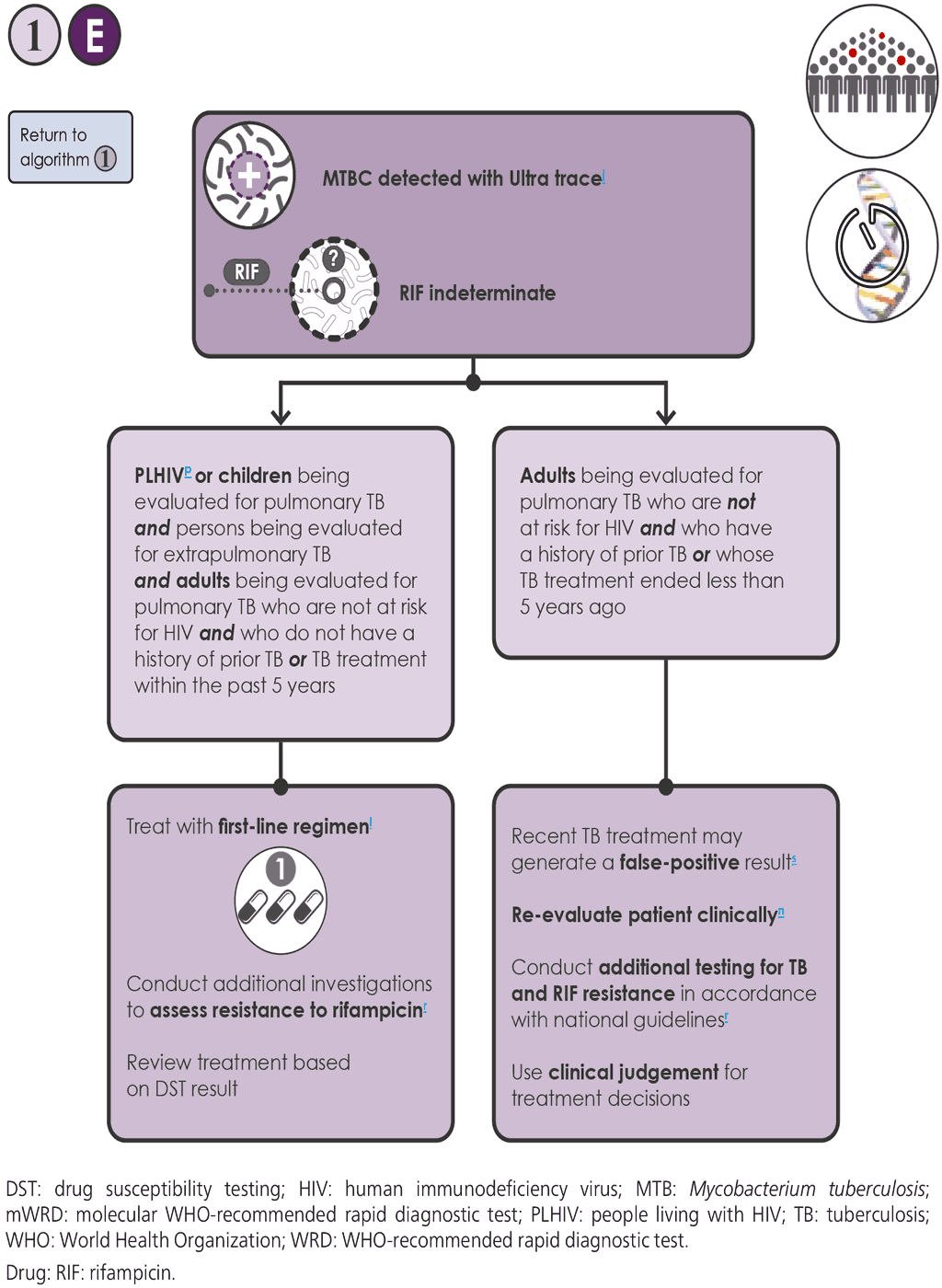
- If the Xpert Ultra test result is “MTBC detected trace” 1E , additional considerations are needed. If concurrent testing with LF-LAM is conducted and the LF-LAM test provides a positive result (i.e. for people living with HIV, children and children living with HIV), an Xpert Ultra “MTBC detected trace” result is considered positive and the person should be initiated on first-line treatment according to WHO and national guidelines.
- WHO suggests not repeating Xpert Ultra testing in adults who have an initial Xpert Ultra trace result to confirm the result, but instead doing the following.
- The person’s clinical characteristics should be reviewed to determine their age, HIV infection status and history of TB treatment, and determine whether the samples are pulmonary or extrapulmonary.
- People may be considered HIV positive if they have tested positive for HIV infection, or have an unknown HIV status but present with strong clinical evidence of HIV infection, reside in settings where there is a high prevalence of HIV or are members of a group at risk for HIV. For all those with unknown HIV status, HIV testing should be performed in accordance with national guidelines.
- Individuals with a history of recent TB treatment include those who successfully completed a course of therapy within the past 5 years. The likelihood of a false positive mWRD result is highest immediately after completing treatment, and slowly declines with time (89, 90). Those who initiated but did not complete therapy and those who failed therapy should be considered as being at high risk of having MDR-TB; careful clinical evaluation is required in such cases.
- A trace positive result in an extrapulmonary sample is considered a true positive and should be treated with first-line treatment.
- Health workers must endeavour to obtain a reliable history of TB treatment, recognizing that some people may not communicate their treatment history because of stigma or, in the case of migrants, concern over legal status.
- For certain populations (e.g. people living with HIV and children who are being evaluated for pulmonary TB; individuals being evaluated for extrapulmonary TB using CSF, lymph nodes and tissue specimens; and adults being evaluated for pulmonary TB, who are not at risk for HIV and who do not have a history of TB treatment in the past 5 years) the following steps should be taken:
- The MTBC detected trace result obtained with the first specimen should be considered as bacteriological confirmation of TB (i.e. a true positive result) and used for clinical decisions.
- The person should be initiated on an appropriate regimen using first-line TB drugs, in accordance with national guidelines, unless the person is at high risk of having MDR-TB (in which case, the person should be initiated on an MDR-TB regimen).
- Additional investigations (e.g. culture and DST) should be undertaken to confirm or exclude resistance to RIF.
- For adults who are being evaluated for pulmonary TB, who are not at risk of HIV and have a history of TB treatment in the past 5 years, the following steps should be taken:
- For adults with a history of recent TB treatment or unknown treatment history, the possibility of the Xpert Ultra trace result being a false positive result should be considered because of the possible presence of non-viable bacilli.
- The person should be re-evaluated and additional testing conducted (including liquid culture) in accordance with national guidelines. The possibility of TB caused by reactivation, relapse or reinfection should be considered.
- In initiating treatment, the clinical presentation and context of the person should be considered, and clinical decisions should be made based on all available information and clinical judgement.
- Further investigations for TB may include CXR, additional clinical assessments and clinical response following treatment with broad-spectrum antimicrobial agents (FQs should not be used).
- Repeat Xpert Ultra testing is of uncertain benefit. A recent WHO GDG recommended against repeat Xpert Ultra testing for individuals with an initial Xpert Ultra trace result for the detection of MTBC.
- Culture and phenotypic DST may be of benefit for detecting TB and drug resistance. The trace result provides no information on RIF resistance.
- Further investigations for TB in children may consider chest x-ray and additional clinical assessments according to the WHO guidelines on management of TB in children (56).
- The person’s clinical characteristics should be reviewed to determine their age, HIV infection status and history of TB treatment, and determine whether the samples are pulmonary or extrapulmonary.
- If the mWRD does not give a result 1F , or gives a result of “error” or “invalid”, the mWRD should be repeated at the same testing site with a second specimen.
Interpretation of discordant results
This algorithm relies on testing of a sample with an mWRD (with or without LF-LAM) to detect MTBC and assess susceptibility to RIF. Discordance in resistance to INH is described in Algorithm 3. On occasion, follow-up testing is recommended to ensure that clinical decisions are well informed. However, discordant results may occur, usually when comparing culturebased results with molecular results. Each discordant result will need to be investigated on a case-by-case basis. General considerations are outlined below.
- For an mWRD result “MTBC detected other than trace”, culture negative (see Point 5 for trace):
- The mWRD result and clinical judgement should be used to guide the treatment decision, pending additional testing.
- The mWRD result should be considered as bacteriological confirmation of TB if the sample was collected from a person who was not recently receiving treatment with anti-TB drugs. Cultures from individuals with pulmonary TB may be negative for several reasons, including that the person is being treated for TB (effective treatment rapidly renders MTBC non-viable), transport or processing problems have inactivated the tubercle bacilli, cultures have been lost to contamination, the testing volume was inadequate, or a laboratory or clerical error occurred.
- Follow-up actions may include re-evaluating the person for TB, reassessing the possibility of prior or current treatment with anti-TB drugs (including FQ use), evaluating response to therapy, and evaluating the possibility of laboratory or clerical error.
- mWRD result “MTB not detected”, culture positive:
- The treatment decision should be based on the culture result. If the person started treatment based on clinical judgement, treatment should be continued and the person recorded as having bacteriologically confirmed TB.
- The culture-positive result should be considered as bacteriological confirmation of TB because culture is the current gold standard for the laboratory confirmation of TB. Using a sputum specimen, WRDs have a pooled sensitivity of 83–90% for detecting pulmonary TB compared with culture (91). Their sensitivity is lower in people living with HIV, children and other specimen types such as CSF.
- False positive cultures can result from a variety of causes, such as cross-contamination in the laboratory (e.g. from inappropriate specimen processing) or sample labelling problems. In well-functioning laboratories, such errors are rare.
- Follow-up actions may include re-evaluating the person for TB, conducting additional testing using mWRDs, culturing additional samples, and evaluating the possibility of laboratory or clerical error. If the person was initiated on anti-TB therapy based on clinical judgement, the response to therapy should be evaluated.
- mWRD result “MTBC detected, RIF resistance detected”; RIF susceptible by phenotypic DST:
- The mWRD result should be used to guide treatment decisions pending additional testing.
- Borderline resistant mutations are known to generate this discordant result, particularly in the BACTEC MGIT system (i.e. a false susceptible phenotypic result). In people infected with strains carrying these mutations treatment with RIF-based first-line regimens often fails (83).
- In some settings with a low prevalence of MDR-TB, silent mutations have been observed that generate a false resistant mWRD result, but these are rare.
- A review of the probe melting temperatures when available (92) or banding pattern on the FL-LPA can aid in determining or inferring the specific mutation (e.g. borderline resistant or silent).
- Follow-up actions may include DNA sequencing, confirmatory testing on another mWRD testing platform, phenotypic DST using solid media, and evaluation of the possibility of laboratory or clerical error.
- mWRD result “MTBC detected, RIF resistance not detected”; RIF resistant by phenotypic DST:
- The treatment regimen should be modified based on the results of the phenotypic DST.
- False RIF-susceptible mWRD results are rare but have been observed in 1–5% of RIF-resistant TB cases tested with the Ultra test in various epidemiological settings. Mutations in the RRDR of the rpoB gene have been shown to account for 95–99% of RIF resistance. The remainder of RIF resistance arises from mutations outside RRDR, which produce an Xpert MTB/RIF result of “RIF resistance not detected”. In settings with a prevalent clone that harbours a mutation outside the RRDR – for example, Eswatini (93) –this may be more common; however, this has not been identified as a major concern in other settings (94). Surveillance to monitor emergence of such clones over time should be considered.
- Follow-up actions may include DNA sequencing, repeating the phenotypic DST, and evaluating the possibility of laboratory or clerical error.
- Xpert Ultra “MTBC detected trace”, culture negative – the interpretation of this result must consider the person’s characteristics, the specimen type and whether the person had been previously treated for TB:
- Cultures may be negative for several reasons, including the person being treated for TB or treated with FQs, transport or processing problems that inactivated the tubercle bacilli, culture contamination or inadequate testing volume, or laboratory or clerical error.
- The small numbers of bacilli in a sample that generates an “MTBC detected trace” result may be due to active TB disease, laboratory cross-contamination, recent exposure to (or infection with) tubercle bacilli (i.e. incipient TB), and current or past treatment for TB.
- The FIND multicentre study revealed that many of the samples that generated results of “MTBC detected trace” and culture negative were from individuals who had completed therapy within the past 4–5 years, presumably because of the presence of small numbers of non-viable or non-replicating bacilli. Thus, “MTBC detected trace” results must be interpreted within the context of prior treatment.
- For people living with HIV and children who are being evaluated for pulmonary TB, or when extrapulmonary specimens are tested, the benefits of the increased sensitivity for the detection of MTBC (i.e. true positives) outweigh the potential harm of decreased specificity (i.e. false positives).
- The “MTBC detected trace” result is considered as bacteriological confirmation of TB (i.e. true positive results) and such people should have been initiated on therapy based on the Xpert Ultra result. The possibility that the culture result was a false negative result should be considered.
- Follow-up actions may include assessing the response to therapy (culture results are often not available for weeks after specimen collection), reassessing the possibility of prior or current treatment with anti-TB drugs (including FQ use), and evaluating the possibility of laboratory or clerical error.
- For adults being evaluated for pulmonary TB who are not at risk of HIV, the balance of benefit and potential harm varies, based on whether the person had been treated previously for TB because of decreased specificity (i.e. false positives).
- For individuals in whom a history of current or prior TB treatment can be reliably excluded:
- Although the “MTBC detected trace” results should be considered as bacteriological confirmation of TB (i.e. true positive results), any clinical decision (e.g. to treat for TB) should be made based on all available laboratory, clinical and radiological information, and clinical judgement.
- The possibility that the culture result was a false negative result should be considered if the samples were collected from a person who was not receiving treatment with anti-TB drugs, because of the paucibacillary nature of the sample. Follow-up actions for people placed on anti-TB therapy may include re-evaluating the person for TB, assessing the response to therapy, reassessing the possibility of prior or current treatment with anti-TB drugs (including FQ use), repeating Xpert Ultra testing, evaluating the possibility of laboratory or clerical error, and repeating the culture (preferably using liquid culture).
- For adults with a history of recent TB treatment:
- The possibility that the Xpert Ultra “MTBC detected trace” result was a false positive result should be considered because of the presence of non-viable bacilli. A culture-negative result is consistent with this possibility.
- If such adults had been initiated on anti-TB therapy based on clinical judgement, follow-up actions may include assessing the response to therapy, conducting additional testing in accordance with national guidelines, repeating culture (preferably using liquid culture), and evaluating the possibility of laboratory or clerical error.
- For adults with a history of recent TB treatment:
- For individuals in whom a history of current or prior TB treatment can be reliably excluded:
- For people living with HIV and children who are being evaluated for pulmonary TB, or when extrapulmonary specimens are tested, the benefits of the increased sensitivity for the detection of MTBC (i.e. true positives) outweigh the potential harm of decreased specificity (i.e. false positives).
- mWRD positive, urine LF-LAM negative:
- An mWRD may give a different result than the urine LF-LAM because the tests have different sensitivities and measure different analytes.
- An mWRD test results of ‘MTBC Detected’ is considered as bacteriologically confirmation of TB. Treatment decisions may be determined following algorithm decision pathways 1B-1E.
- Urine LF-LAM positive, mWRD negative:
- The LF-LAM test may give a different result than an mWRD or culture. This is not unexpected because the tests have different sensitivities and measure different analytes.
- A positive LF-LAM result is considered is considered as bacteriological confirmation of TB. Treatment decisions should rely on clinical judgement and all available information, including (but not limited to) chest x-ray findings (if available) and any other available bacteriological results.
- When LF-LAM results are consistently positive, without positive LC-aNAAT results, investigation of the quality of testing and local epidemiology of non-tuberculosis mycobacteria and extrapulmonary TB in the tested population is warranted to understand the difference.

 Обратная связь
Обратная связь