Liens transversaux de livre pour PLATEFORME DE PARTAGE DES CONNAISSANCES SUR LA TUBERCULOSE DE L'OMS
2.5.1 Phenotypic DST
Treatment of TB has undergone significant changes over recent years, with new drugs and regimens being recommended; hence, the definitions for DR-TB have been revised accordingly. The updated definitions are as follows (26):
- pre-XDR-TB is “TB caused by M. tuberculosis strains that fulfil the definition of MDR/RR-TB and that are also resistant to any FQ”; and
- XDR-TB is “TB caused by M. tuberculosis strains that fulfil the definition of MDR/RR-TB and that are also resistant to any FQ and at least one additional Group A drug (i.e. BDQ or LZD)”.
These changes have important implications for Member States, particularly for the scaling-up of the detection of resistance to FQs and BDQ. In addition, there is an increasing demand for DST for other new and repurposed drugs.
Indirect phenotypic DST on solid (LJ, 7H10 agar, 7H11 agar) and liquid media (7H9 broth, BACTEC Mycobacterial Growth Indicator Tube [MGIT] system) is reliable and reproducible, and it remains the reference standard for many anti-TB medicines (27). The DST manual in Web Annex C collates the WHO recommendations for phenotypic and genotypic DST, and includes the critical concentrations for Pa and Cs that were established in 2023 (Box 2.11). Reliable phenotypic DST methods are available for RIF, INH, FQs, PZA, BDQ, LZD, AMK, STR CFZ, DLM, Pa and Cs. Phenotypic DST is currently not recommended for EMB, ETO, prothionamide, para-aminosalicylic acid (PAS), imipenem-cilastatin and meropenem (MPM). The manual also provides information on sources of pure powders for phenotypic DST, detailed methods for preparing drug-containing media, interpretation and reporting of results, and quality control (QC).
The new definition of XDR-TB requires DST results for FQs, BDQ and LZD; thus, testing for resistance to BDQ and LZD has become a priority, particularly testing for resistance to BDQ. Phenotypic DST for BDQ and LZD can be performed using either the MGIT or Middlebrook 7H11 media. Lyophilized vials containing BDQ powder for use with MGIT are manufactured by Becton Dickinson and are available in the Global Drug Facility catalogue (29). In addition, the BDQ pure drug substance for use in phenotypic DST is provided free through the BEI Resources of the US National Institutes of Health (NIH) (30); however, courier costs need to be covered. An information note that explains the request process is also available (31). The BEI Resources also provides DLM and Pa. LZD powder is available from Sigma (PZ0014–5MG) or Cayman Chemical (CAS 65800–03–3). The DST manual in Web Annex C provides specific details on the sources of the drug powders used for phenotypic DST.
It is essential to monitor all new batches of drug solutions to detect challenges with quality or performance as early as possible. It may not be enough to test only one reference strain at the CC because of possible variations in the isolates. A more robust way to control for variations is to include multiple dilutions above and below the CC, to ensure that the reference strain used is behaving as expected.
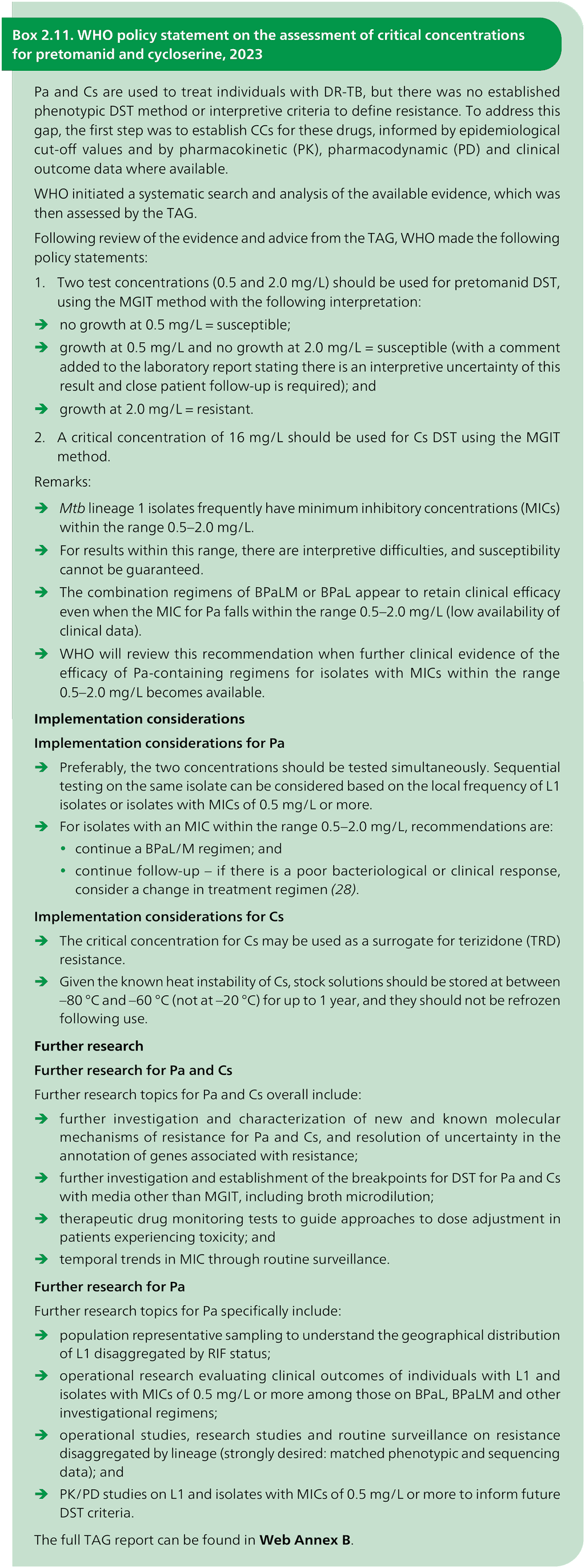
The latest CCs for all drugs are listed in Table 2.9 and Table 2.10; they were adapted from Tables 1 and 2 of Web Annex C.
Table 2.9. Critical concentrations for first-line medicines recommended for the treatment of drug-susceptible TB
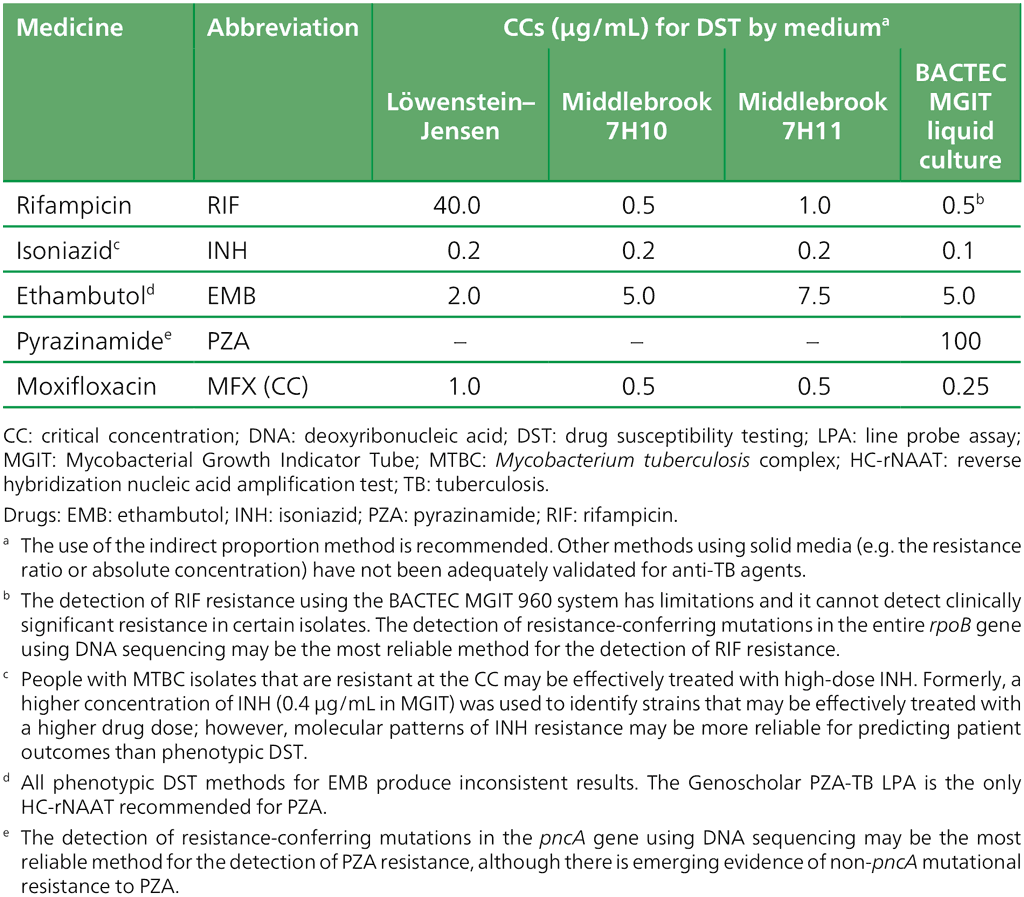
Table 2.10. Critical concentrations and clinical breakpoints for medicines recommended for the treatment of MDR/RR-TB
CB: clinical breakpoint; CC: critical concentration; DST: drug susceptibility testing; LJ: Löwenstein–Jensen media; MDR/RR-TB: multidrug- or rifampicin-resistant tuberculosis; MGIT: Mycobacterial Growth Indicator Tube.
Drugs: AMK: amikacin; CS: cycloserine; DLM: delamanid; LFX: levofloxacin; MFX: moxifloxacin; PZA: pyrazinamide; STR: streptomycin; TRD: terizidone.
a LFX and MFX CCs for LJ were established despite very limited data.
b CB concentration for 7H10 and MGIT apply to high-dose MFX (i.e. 800 mg daily).
c The CC for CS may be used as a surrogate for Tad resistance.
d DST is not reliable and reproducible; hence, DST is not recommended.
e DLM should be stored away from light and heat, as per the manufacturer’s materials safety data sheet.
f PZA is only counted as an effective agent when DST results confirm susceptibility in a quality-assured laboratory.
g AMK and STR are only to be considered in case of rescue regimens or individualized treatment, and only if DST results confirm susceptibility.
h No growth at 0.5 = susceptible; growth at 0.5 and no growth at 2.0 = susceptible, but with a comment on uncertainty; growth at 2.0 = resistant.
Source: adapted from Table 3 in Web Annex C.
2.5.2 Whole-genome sequencing
Phenotypic DST remains the reference standard for most anti-TB compounds; however, this method is slow, and it requires specialized infrastructure and highly skilled staff. Genotypic DST (also referred to as molecular DST) holds promise to overcome some of the obstacles of phenotypic DST, and complements phenotypic DST as a reference standard when testing for RIF, EMB and PZA resistance. Currently, the available mWRDs for DST can be used to detect specific mutations known to confer phenotypic resistance. Rapid molecular tests for resistance to RIF, INH and FQ can be implemented in decentralized settings; such tests can deliver rapid results to inform the selection of the initial treatment regimen while awaiting follow-on DST for other anti-TB drugs.
DNA sequencing using NGS technologies is a method for the detection of mutations associated with drug resistance for many anti-TB drugs (32). NGS-based DST could reduce the need for phenotypic DST for patient-care decisions; it may be particularly useful for drugs for which phenotypic DST is unreliable or for settings that do not have the capacity to perform phenotypic DST.
NGS refers to techniques that rely on the sequencing of multiple DNA fragments in parallel, followed by bioinformatics analyses to assemble the sequences. The technologies can be used to determine the nucleotide sequence of an entire genome (i.e. WGS) or part of a genome (i.e. targeted NGS) in a single sequencing run. WGS and targeted NGS are recommended for use in the surveillance of DR-TB and for DST.
WGS is a powerful tool for detecting mutations and it serves as a reference test for confirming mutations; however, it depends on having a culture isolate and is impacted by delays in growing the organism (33). The WHO mutation catalogue (23) provides a standardized methodology for the analytical pipeline for analysis of NGS results and interpretation of mutations. The tools can be used both for WGS and targeted NGS.
Phenotypic DST remains important for classifying resistance, particularly for new drugs where resistance is not fully elucidated. As more phenotypic DST data are matched with WGS data in the catalogue, the performance of the catalogue and the utility of WGS for new drugs will improve. Contributors are encouraged to upload data to the TB sequencing portal (34).
Use of WGS and phenotypic DST is now the reference standard for RIF and PZA owing to the limitations of phenotypic DST alone for these drugs.
Section 2.4.4 describes the targeted NGS tests for the detection of resistance to anti-TB medicines recently recommended by WHO for use directly on clinical samples. These tests can detect mutations associated with resistance to RIF, INH, PZA, EMB, FQ, BDQ, LZD, CFZ, AMK and STR.
Table 2.11 presents an overview of the WHO-recommended diagnostic approaches, reference methods and clinical interpretation for anti-TB medicines.
Table 2.11. WHO-recommended diagnostic approaches, reference methods and clinical interpretation for anti-TB medicines
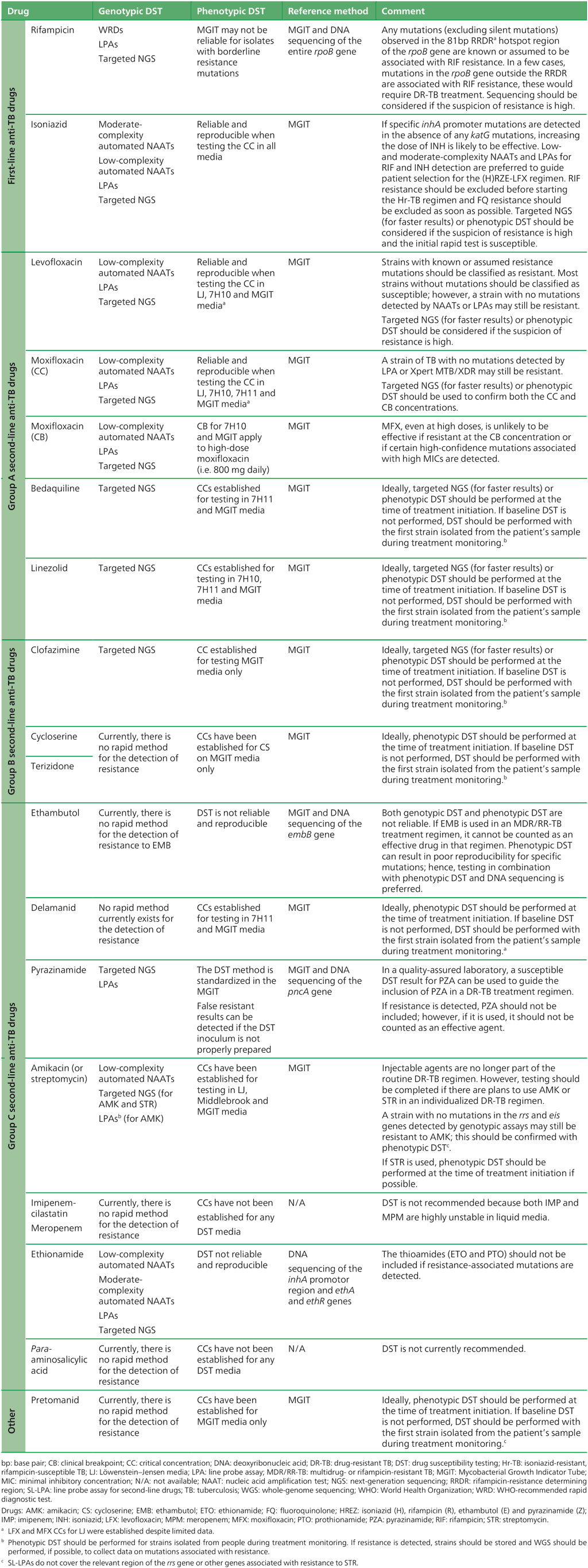
Source: adapted from Table 3 in Web Annex C.

 Retour
Retour