Liens transversaux de livre pour 1266
The design of longer regimens (18–20 months) is founded on grouping of medicines recommended for use in longer regimens based on the drug-resistance profile (Table 2.6.1).
In ideal conditions, only a small proportion of MDR/RR-TB patients should opt for longer regimens, because this indication is mainly for those who cannot benefit from either BPaLM/BPaL or the 9-month all-oral regimen. Reasons for not using the shorter regimens may be related to the age of the patients, additional resistance (including FQ resistance and other Group A medicines; i.e. XDR-TB), intolerance to key medicines used in shorter regimens, severity of disease, pregnancy, certain types of extrapulmonary TB or other complications needing an individualized approach.
Under many of these circumstances, only less potent and more toxic drugs are left to be used for treatment and lengthy regimens are therefore needed to cure without relapse. Longer regimens, especially if clinical conditions are complex (e.g. advanced disease with higher burden of bacilli and severe disease affecting critical organs) are usually associated with higher likelihood of toxicity, owing to factors such as longer drug exposure, higher intolerance, adverse effects and greater potential for DDIs in critically ill patients.
All these conditions that may lead to less patient-friendly regimens with higher pill burden and toxicity can increase the likelihood of unfavourable treatment outcomes such as treatment failure, LTFU and death. All DR-TB patients need a patient-centred approach with treatment adherence support and aDSM, but in longer regimens these activities become more crucial. Patients will need support to overcome the hardships associated with TB and its treatment, including daily adherence challenges, adverse drug reactions, indirect costs and stigma.

6.1 Eligibility
A longer treatment regimen should be proposed mainly when the BPaLM/BPaL or 9-month all-oral regimen cannot be used. Section 4 and Section 5 discuss the eligibility criteria for these shorter regimens.
A longer regimen is expected to be used in the following situations:
- severe extrapulmonary TB;
- additional resistance to key medicines of the BPaLM/BPaL regimen (except moxifloxacin) or the 9-month all-oral regimen;
- lack of response to shorter treatment regimens (e.g. treatment failure due to no bacteriological conversion, no clinical response, emerging resistance or LTFU);
- drug intolerance to the component medicines of the BPaLM/BPaL regimen (except moxifloxacin) or 9 months shorter all-oral treatment regimen; and
- pregnant and breastfeeding women who could not benefit from the 9-month shorter all-oral regimen owing to certain clinical conditions or children aged below 14 years who could not be treated with BPaLM/BPaL or who, for any reason, cannot opt for a 9-month regimen.
There is limited or no evidence of BPaLM/BPaL use in some patient groups; thus, a longer regimen could also be considered as an option for patients with low BMI (<17 kg/m² ), altered hepatic enzymes (3 times greater than the upper limit of normal), baseline anaemia (Hb <8 g/dL), thrombocytopenia (platelet count <150 000/mm³ ) or preexisting peripheral neuropathy of Grade 3–4 (23, 75, 76).
Any patient eligible for a longer regimen should undergo a pretreatment assessment to optimize the drug selection, reduce the chances of AEs and thus increase the probability of the favourable treatment outcomes. The pretreatment assessment includes:
- a detailed clinical history (including all comorbidities, medications and known intolerances), a physical examination, a blood test, chest X-ray or other imaging and bacteriological tests; and
- a list of current effective TB medicines available based on a clinical history of drugs taken before this treatment episode and guided by the DST results or sequencing of the most recent sample from the patient (or the index case).
In addition to the eligibility criteria and preclinical assessment, a clinician should also consider:
- development of a personalized treatment approach (patient-centred approach) and close follow-up, including food support if needed, to increase bioavailability of drugs, improve nutritional status and facilitate adherence;
- provision of advice on contraception for women of childbearing age;
- availability of ancillary medications (e.g. corticosteroids in the case of disseminated TB or TB meningitis or pericarditis, pretreatment blood transfusion in the case of severe anaemia and nutritional support) and other interventions (e.g. intravenous [IV] medication in the case of severe malnutrition and malabsorption, insertion of peripherally inserted central catheter, or surgery in the case of restricted options and meeting criteria for intervention); and
- provision of counselling, depending on the patient’s comorbidities (e.g. HIV or diabetes) or preexisting conditions needing to be treated to optimize TB treatment outcomes.
6.2 Composition and duration of the regimens
When designing longer regimens, several basic principles need to be respected, in line with the best available evidence on composition of the regimens, as per the recommendations listed below.

6.2.1 Choice of components for the longer MDR-TB regimens
A stepwise approach guides the design of longer MDR-TB regimens (Table 2.6.1).
The selection of medicines follows a priority order based on the revised classification of regimen components, and a fully oral regimen is preferred. At least four drugs must be selected, starting from Group A and then from Group B. Group C drugs are usually included in a longer regimen if it cannot be composed with Group A and B agents alone. The choice of drugs from Group C is usually determined by the order in which the medicines are ranked, and by the individual circumstances of the patient and the setting. A recent review of the observational data found no additional safety concerns when bedaquiline was used for longer than 6 months; however, no clear evidence was available to indicate whether longer use added efficacy (1). The clinicians may therefore consider continuing bedaquiline for longer than 6 months and adding some flexibility for regimen design and the number of effective drugs.
In the case of longer treatment regimens, an individual approach is needed. Therefore, apart from the drug classification, it is crucial to optimize drug selection according to the patient’s clinical condition and the drug-resistance pattern. Considerations include:
- the clinical history of drugs taken in the past by the patient or the index case, or according to local resistance epidemiology in the country or region;
- the DST results – where available, is it of utmost importance to guide the drug selection using phenotypic or genotypic DST; in patients with extensive patterns of resistance, whenever possible, it is advised to perform whole genome sequencing; and
- selecting drugs according to their special features – in addition to susceptibility, key drug features and clinical particularities of the patient that may boost survival must be considered (e.g. likelihood of effectiveness, CNS penetration, drug–drug interaction profile, tolerance and patient preference, oral absorption and bioavailability).
Most anti-TB drugs are used once daily to achieve a high peak serum concentration that increases the bactericidal and sterilizing effect and to support adherence (to avoid missed or partial doses). The doses of anti-TB drugs by weight bands are outlined in the Annex 4. The essential information about TB medicines used in MDR/RR-TB treatment is described in detail in Annex 1.
Many patients may have comorbidities and AEs that need to be addressed separately. Hospitalization, surgery and other adjuvant treatment may be needed at certain stages of treatment. Comprehensive monitoring and treatment adherence support are important to ensure a favourable treatment experience. Access to palliative and end-of-life care services may be needed, with a patient-centred approach to relieve the suffering from the disease and its treatment (77). Respiratory infection control measures at the sites where the patient is being treated, contact tracing and counselling are important accompanying measures for clinical care and public health.
Table 2.6.2 summarizes some common situations that a clinician may face, and the decisions that could be taken to adjust the treatment regimen accordingly. The suggested regimens may vary based on the individual clinical circumstances and the availability of medicines. Table 2.6.2 is not exhaustive. Although it is recommended to use at least four effective agents initially, not all the regimens composed using this algorithm have been tested directly in either research or field conditions. Moreover, when Group C agents are included, the number of medicines in the regimen may exceed four, to reflect the uncertainty about the efficacy of some of these medicines. In such situations, the advice of a specialist is important to ensure the safest and most effective possible regimen.
6.2.2 Medicines used in longer MDR-TB treatment regimens
The classification of medicines used in MDR/RR-TB treatment regimens was revised following the evidence-informed update of the WHO guidelines on DR-TB treatment in 2018. TB medicines to be used for treatment of MDR/RR-TB are categorized into Groups A, B and C (Table 2.6.1) (1). This classification is based on drug class and level of certainty in the evidence on effectiveness and safety (i.e. balance between benefits and risk of harm). The data analysed relate mainly to adult patients who received regimens in recent years. Groups A–C feature the medicines to be used to compose longer MDR-TB regimens. WHO considers that, under programmatic conditions, only these medicines (Groups A–C) have a role in longer MDR-TB treatment regimens. In addition to agents from Groups A–C, the potential role for clavulanic acid and high-dose isoniazid was discussed (see “Other medicines” in this section).
The most notable differences between the classification of longer regimen components used before 2018 and the current guidelines are an upgrade in the priority of bedaquiline, linezolid, clofazimine and cycloserine/terizidone; placement of delamanid in Group C; and lowering of priority for pyrazinamide, amikacin, streptomycin, ethionamide/prothionamide and p-aminosalicylic acid, relative to other treatment options. Several agents that were featured previously in these groups are no longer included because they are:
- no longer recommended (e.g. ofloxacin, capreomycin and kanamycin);
- rarely used in longer regimens (e.g. high-dose isoniazid); or
- an adjunct agent that is not intended to be used alone (e.g. clavulanic acid is used only in combination with the carbapenems).
The classification facilitates design of the treatment regimen for patients with DR-TB who are not eligible for the BPaLM/BPaL or 9-month treatment regimens. Table 2.6.1 summarizes the general steps to take when including agents for the longer MDR-TB regimen according to the latest WHO guidance, with more details provided for some of the most common situations and patient subgroups that clinicians and NTPs may encounter.
Table 2.6.1. Grouping of medicines recommended for use in longer MDR-TB regimensᵃ
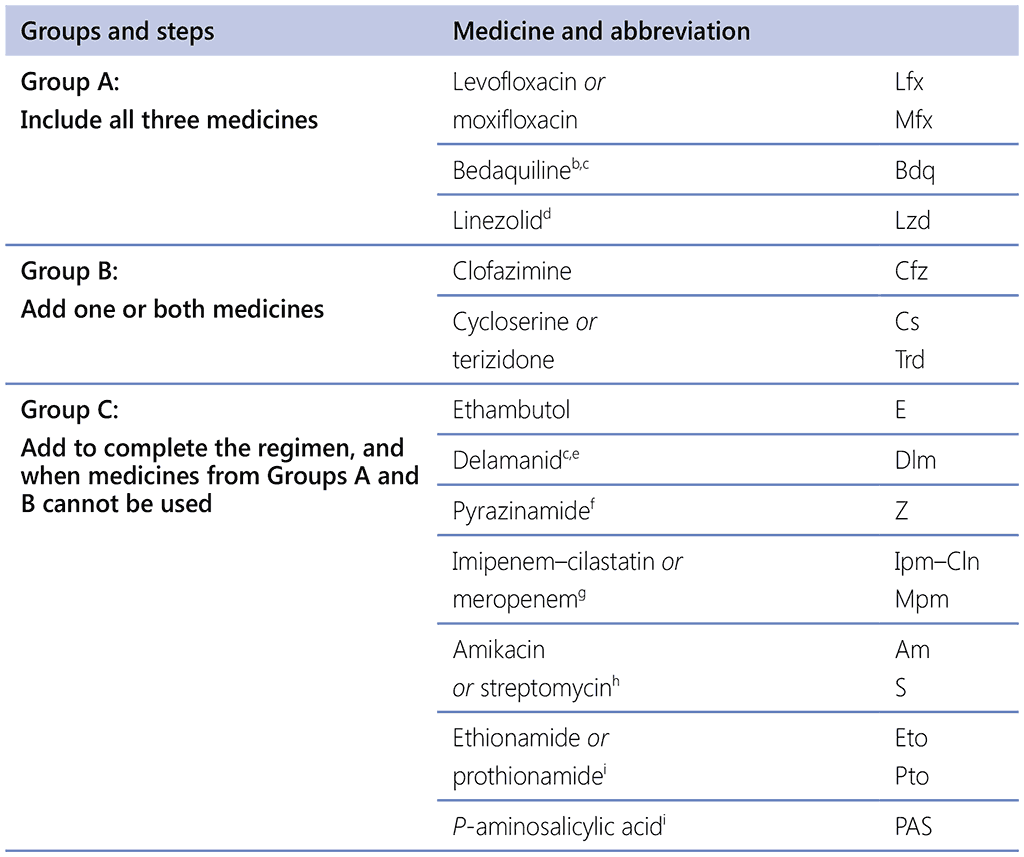
DST: drug susceptibility testing; ECG: electrocardiography; GDG: Guideline Development Group; IPD: individual patient data; LPA: line probe assay; MDR-TB: multidrug-resistant TB; TB: tuberculosis.
a This table is intended to guide the design of individualized, longer MDR-TB regimens. Medicines in Group C are ranked by decreasing order of usual preference for use, subject to other considerations. The 2018 IPD meta-analysis for longer regimens included no patients on thioacetazone and high-dose isoniazid, for a meaningful analysis. No recommendation on perchlozone, interferon gamma or sutezolid was possible owing to the absence of final patient treatment outcome data from appropriate studies (1).
b Bedaquiline is usually administered 400 mg orally once daily for the first 2 weeks, followed by 200 mg orally thrice weekly for 22 weeks (total duration of 24 weeks). As a result of multiple reviews following new data gradually becoming available, the use of bedaquiline is not restricted by age of the patient. Evidence on the safety and effectiveness of bedaquiline use beyond 6 months was insufficient for review in 2018. Therefore, the use of bedaquiline beyond 6 months was implemented following best practices in “off-label” use (78). New evidence on the safety profile of bedaquiline use beyond 6 months was available to the GDG in 2019, but the GDG was not able to assess the impact of prolonged bedaquiline use on efficacy, owing to the limited evidence and potential residual confounding in the data. However, the evidence supports the safe use of bedaquiline beyond 6 months in patients who receive appropriate schedules of baseline and follow-up monitoring. The use of bedaquiline beyond 6 months still remains as off-label use and, in this regard, best practices in off-label use still apply.
c Evidence on the concurrent use of bedaquiline and delamanid was insufficient for review in 2018. In 2019, new evidence on both the safety and effectiveness of concurrent use of bedaquiline and delamanid was made available to the GDG. In relation to safety, the GDG concluded that the data suggested no additional safety concerns regarding concurrent use of bedaquiline and delamanid. More evidence was added to that regard between 2020 and 2022 (79). Both medicines may be used concurrently in patients who have limited other treatment options available to them, and if sufficient monitoring (including baseline and follow-up ECG and electrolyte monitoring) is in place. The data on the effectiveness of concurrent use of bedaquiline and delamanid were reviewed by the GDG in 2019, but owing to the limited evidence and potential residual confounding in the data, the GDG could not proceed with a recommendation on effectiveness (1).
d Use of linezolid for at least 6 months was shown to increase effectiveness, although toxicity may limit its use. The analysis suggested that using linezolid for the whole duration of treatment would optimize its effect (about 70% of patients on linezolid with data received it for more than 6 months, and 30% for 18 months or the whole duration). No patient predictors for early cessation of linezolid could be inferred from the IPD subanalysis.
e Evidence on the safety and effectiveness of delamanid beyond 6 months was insufficient for review. The use of delamanid beyond these limits should follow best practices in “off-label” use (78). As a result of multiple reviews following new data gradually becoming available throughout the years the use of delamanid is not restricted by age of the patient.
f Pyrazinamide is only counted as an effective agent when DST results confirm susceptibility.
g Every dose of imipenem–cilastatin or meropenem should be preceded by the oral administration of oral clavulanic acid 30–60 minutes beforehand; oral clavulanic acid is only available in formulations combined with amoxicillin. Amoxicillin–clavulanic acid is not counted as an additional effective TB agent and should not be used without imipenem–cilastatin or meropenem.
h Amikacin and streptomycin are only to be considered if DST results confirm susceptibility and high-quality audiology monitoring for hearing loss can be ensured. Streptomycin is to be considered only if amikacin cannot be used (unavailable or documented resistance) and if DST results confirm susceptibility (streptomycin resistance is not detectable with second-line molecular LPAs and phenotypic DST is required). Kanamycin and capreomycin are no longer recommended for use in MDR-TB regimens.
i These agents only showed effectiveness in regimens without bedaquiline, linezolid, clofazimine or delamanid and are thus only proposed when other options to compose a regimen are not possible.
Group A
Group A includes FQ (levofloxacin and moxifloxacin), bedaquiline and linezolid. These medicines were found to be highly effective in improving treatment outcomes and reducing deaths in the evidence reviewed in 2018 for the WHO guidelines (1), and it is strongly recommended that they be included in all longer MDR-TB regimens and used for all MDR/RR-TB patients eligible for longer regimens unless there is a toxicity issue or drug resistance.
Levofloxacin and moxifloxacin
Levofloxacin and moxifloxacin are later-generation FQ, and their use in the meta-analysis that informed the WHO guidelines (2018 update) resulted in a significantly lower risk of treatment failure or relapse and death (1, 8, 80, 81). Levofloxacin and moxifloxacin appear to be equally effective in FQ-susceptible patients receiving longer regimens, and either of these drugs can be considered for MDR/RR-TB treatment using these regimens. Ciprofloxacin and ofloxacin are less effective in MDR-TB treatment and are no longer recommended.
Reliable rapid molecular DST is available for levofloxacin and moxifloxacin (including Xpert MTB/XDR and second-line LPA). Not all point mutations present the same resistance profile. Despite some mutations having consistently high minimum inhibitory concentrations (MICs) (i.e. gyrA D94N or D94Y), most mutations present a range of phenotypic resistance that may cross critical concentration (CC) and clinical breakpoint (CB) levels. Therefore, once FQ resistance has been detected by molecular methods and treatment has started, a phenotypic method may be used as a reference test for distinguishing between high-level (>CB) and low-level (>CC and <CB) resistance mutations, possibly allowing for the use of a high-level FQ dose. Where these mutations are detected, the composition of the longer regimen should be re-evaluated based on phenotypic DST results at the CB (82).
If DST for moxifloxacin confirms high-level resistance, or if the patient’s history suggests that moxifloxacin has not been effective (e.g. if used in a failing regimen for more than 15–30 days), moxifloxacin should not be used. Work is ongoing to optimize the use of moxifloxacin related to sequencing, CC in phenotypic DST and clinical correlation (82–84).
Bedaquiline
In the IPD meta-analysis used as evidence for the WHO guidelines, bedaquiline use resulted in significantly fewer episodes of treatment failure, relapse and death (1). There is growing experience of its use in children, adolescents and older people, patients with extrapulmonary TB disease and PLHIV (85, 86). Currently, there is no age restriction for the use of bedaquiline, including in longer regimens (56).
Analyses of observational study data highlighted the improved survival of patients treated with regimens containing bedaquiline (56) and the favourable safety profile of bedaquiline when the drug is used alongside other TB medicines, including medicines with a QT prolongation effect (e.g. moxifloxacin, clofazimine and delamanid) (87–92). The recent data review for the WHO consolidated guidelines (1) suggested no additional safety concerns for the use of bedaquiline beyond 6 months, used concurrently with delamanid or in pregnancy (89). The available data suggested that the concurrent use of bedaquiline and delamanid does not increase the risk of clinically meaningful QT prolongation (93).
Some inconclusive evidence is emerging; for example, some published data on the rapid advent of bedaquiline resistance in settings where it is used may suggest a possibility of bedaquiline being a low genetic barrier drug (i.e. causing resistance to emerge rapidly) as a result of frequent natural mutations. Also worth considering is the long half-life of the drug (5.5 months), which may lead to the drug acting as monotherapy in patients lost to follow-up. FQ resistance testing should be performed to prevent bedaquiline resistance acquisition, and the levels of resistance should be monitored when possible. Bedaquiline presents cross-resistance with clofazimine in cases of Rv0678 gene mutation (which lead to upregulation of efflux pumps) and pepQ mutations. Resistance may occur spontaneously, even without prior exposure to bedaquiline or clofazimine (4.1% in some studies) (94, 95). Mutations at the atp-E gene may confer high-level resistance to bedaquiline.
Linezolid
Linezolid has shown anti-TB activity in vitro and in animal studies, and its effectiveness in humans was demonstrated in the meta-analysis conducted for the WHO guidelines, as well as in recent trials involving XDR-TB patients (1, 96–100).
Linezolid is associated with considerable toxicity, which necessitates close monitoring for signs of bone marrow suppression and neuropathies. The 2018 IPD meta-analysis informing the WHO guidelines included information from more than 300 patients who were treated with linezolid for at least 1 month, mostly on 600 mg daily. About 30% of patients received linezolid for 1–6 months, but over 30% received it for more than 18 months, and these patients had the lowest frequency of treatment failure, LTFU and death. This analysis also suggested that the optimal duration of use would be about 20 months, corresponding to the usual total duration of a longer MDR-TB regimen; however, the analysis did not account for survivorship bias (i.e. that those who complete the full length of treatment are more likely to have a successful outcome, given that deaths and losses to follow-up occur earlier) (1, 101).
The evidence from the WHO consolidated guidelines (1) suggests that linezolid should be used for as long as it is tolerated. There may be improved outcomes if linezolid is used for the full duration of treatment. However, it probably has its greatest added effect (including protection of other second-line drugs against acquired drug resistance) during the first months of treatment when the bacillary load is highest (102). If toxicity develops, dosing of linezolid should be reduced or replaced by another bactericidal drug (15).
Linezolid is not affected or metabolized by the cytochrome p450; however, it is an inhibitor of monoamine oxidase (IMAO), leading to an increase in serotonin and tyramine levels. Serotonergic syndrome, which can be serious and life threatening, can result when linezolid is given concomitantly with other IMAO drugs that are often used in clinical practice in TB patients (e.g. antidepressants, opioid pain killers such as tramadol, common cold medications or antitussives such as dextromethorphan) (103).
Group B
Group B medicines include clofazimine and cycloserine or terizidone, which were found to be effective in improving treatment outcomes but limited in reducing deaths in the evidence reviewed in 2018 for the WHO guidelines (1). One or both drugs can be added to ensure that a longer regimen starts with at least four effective medicines.
Clofazimine
Clofazimine is an antileprosy medicine that has shown in vitro activity against Mtb and has been used as a second-line TB medicine for several years. The meta-analysis conducted for the WHO guidelines reinforced the evidence for the effectiveness and safety profile of clofazimine (1). When used with drugs that prolong the QT interval (e.g. bedaquiline, FQ and delamanid), clofazimine may cause additive QT prolongation. ECG monitoring should be implemented when several QT-prolonging drugs are also part of the regimen. Non-TB drugs that cause QT prolongation should be avoided if possible.
Common AEs associated with clofazimine are brown-orange or purple-red discolouration of skin, conjunctiva, cornea and body fluids; dry skin, pruritus, rash, ichthyosis and xerosis; gastrointestinal intolerance; and photosensitivity. Patients should be well informed from the outset of the reversible skin colour changes that occur in most patients. The orange-brown skin changes are reversible within a few months (sometimes more) of the drug being stopped and are not considered dangerous. These skin changes can be quite concerning to patients and reassurance is required. Clofazimine can be used during pregnancy or breastfeeding owing to limited data and to pigmentation of the infant if the drug is used during breastfeeding. Clofazimine is partially metabolized by the liver; hence, caution or adjustment of the dose is required for patients with severe hepatic insufficiency.
Cycloserine
Cycloserine is a bacteriostatic drug that inhibits cell wall synthesis, and it has no known cross-resistance to other TB medicines. Terizidone (composed of two molecules of cycloserine) may be used instead of cycloserine, and cycloserine and terizidone are considered interchangeable. Because of difficulties in interpreting DST (there is no reliable genotypic or phenotypic DST for cycloserine or terizidone), cycloserine or terizidone should only be considered when other criteria of likelihood of effectiveness are met; for example, any reliable evidence on population levels of drug resistance, and prior use of cycloserine or terizidone based on a reliable clinical history (Section 3.1). Patients should be well informed of the potential AEs of cycloserine. A major drug AE is CNS toxicity, including inability to concentrate, depression, behaviour change (e.g. violence and aggressiveness, and suicidal ideation), frank psychosis, seizures and lethargy.
Cycloserine may exacerbate preexisting neurologic or psychiatric conditions. Situations of stigma, extreme poverty and social vulnerability are not infrequent among MDR/RR-TB patients, and these also affect mental health. Depression and anxiety are also highly prevalent and can lead to a worse prognosis and LTFU, especially in programmes without patient-centred systems. In these situations, management of cycloserine toxicity is critical to obtain good clinical outcomes and to avoid serious AEs.
Group C
Group C comprises both TB and repurposed medicines that are positioned at a lower priority than the Group A and B agents, either because they are less effective (ethambutol, delamanid, pyrazinamide, ethionamide/prothionamide and p-aminosalicylic acid) or because they are more toxic and cumbersome to administer parenterally (imipenem–cilastatin, meropenem, amikacin and streptomycin). These drugs are usually included in a longer regimen if it cannot be composed with Group A and B agents alone.
Ethambutol
Ethambutol is a TB medicine that is used in the treatment of DS-TB and may be added to longer MDR-TB regimens. At recommended dosages, the safety profile of ethambutol is good. Owing to difficulties in interpreting its DST, ethambutol should only be considered when other criteria of likelihood of effectiveness are met (e.g. evidence on a population level of low prevalence of drug resistance in circulating MDR/RR-TB strains and no prior use of ethambutol based on a reliable clinical history).
Delamanid
Based on current evidence on its effectiveness and safety, delamanid is recommended for use as a Group C agent (1). Delamanid has a potent in vitro bactericidal activity and potential sterilizing activity; it is thought that nitroimidazooxazole derivatives generate reactive nitrogen species, including nitrogen oxide, which are responsible for cell poisoning in low metabolic states. There is no age restriction for use of delamanid and there are currently dispersible formulations that are preferred over crushing and dispersing adult tablets (56, 104). Delamanid is strongly bound to plasma proteins, resulting in low CNS penetration; however, studies in humans and animals with CNS TB suggest that delamanid could potentially play a beneficial role when other options are not available (105).
The recent data review for the WHO guidelines (1) suggested that there are no additional safety concerns for concurrent use of delamanid with bedaquiline. The combined QT effects, compared with bedaquiline or delamanid alone, were evaluated in an RCT of 75 patients (>3000 ECGs) (90). Studies undertaken between 2020 and 2022 had shown no increased toxicity with the use of delamanid beyond 6 months; they showed safety on the concomitant use of delamanid with bedaquiline, while increasing rates of survival of patients with restricted therapeutic options (79, 93).
Animal data show no evidence of teratogenicity. Although the case series of pregnant women on delamanid are small, all children had excellent birth outcomes, suggesting that pregnant women in need should not be denied access to delamanid. It can be considered for the treatment of DR-TB in pregnant women with restricted therapeutic options (106).
Pyrazinamide
Pyrazinamide has been routinely added to MDR-TB regimens except where there is a reasonable clinical contraindication for its use (e.g. hepatotoxicity), or other serious AE or drug resistance. However, reliable DST for pyrazinamide is not widely accessible; hence, this drug has often been used without DST or regardless of documented resistance. In the longer regimens, pyrazinamide is recommended for inclusion only when DST results confirm susceptibility (in such cases it is counted as one of the effective agents); in any other cases, if pyrazinamide is included in the regimen, it is not counted as one of the four effective drugs (107, 108). There are synergies between pyrazinamide and other medicines such as bedaquiline, through complex mechanisms of action targeting dormant bacteria.
Imipenem–cilastatin and meropenem
Imipenem–cilastatin (not used in patients aged <15 years) and meropenem are the only carbapenems that have an established role in MDR-TB regimens. They are administered intravenously – a major drawback that limits their more widespread use outside hospitals, especially in resource-constrained settings (109–113). Daily IV injections are not usually feasible unless there is a surgically fitted port (a port-a-cath) or a peripherally inserted central catheter connected to a major vein. Meropenem with clavulanate as part of regimens (usually also containing linezolid) for patients with MDR-TB and XDR-TB has been shown to improve culture conversion and survival (114–116). Clavulanic acid (as co-amoxyclav) is not a TB medicine but is an adjunct agent that is given orally each time a carbapenem dose is administered, about 30 minutes before the IV infusion. When included in a regimen, clavulanic acid is not counted as one of the TB agents, and it should not be used without the carbapenem.
Amikacin and streptomycin
Amikacin and streptomycin are the only two aminoglycoside antibiotics that can be used when options for composition of the treatment regimen are limited. Based on the evidence reviewed in 2018, amikacin and streptomycin were associated with lower rates of treatment failure or relapse and death when used in people with Mtb strains susceptible to amikacin or streptomycin. However, these drugs share the disadvantages and serious toxicities (i.e. ototoxicity and nephrotoxicity) of other injectable agents that are no longer recommended (i.e. kanamycin and capreomycin). Given the high frequency of streptomycin resistance in patients with MDR/RR-TB in many settings, and its extensive historical use as part of older first-line TB regimens in many countries, streptomycin is unlikely to have much use in MDR-TB regimens.
Ethionamide and prothionamide
In WHO guidance, ethionamide and protionamide are considered interchangeable. The WHO consolidated guidelines make a conditional recommendation against their use in longer MDR-TB regimens, reserving them for situations where multiple, more effective agents (e.g. bedaquiline, linezolid and clofazimine) cannot be used. Apart from the low bactericidal profile, use of ethionamide and prothionamide is limited because of poor gastrointestinal tolerance, which could be potentially linked to bad adherence. In pregnant women, these drugs are usually not recommended owing to poor tolerance, decrease in thyroid-stimulating hormone (TSH) levels (which are fundamental for the development of the fetus) and concerns raised by effects in animal reproductive studies.
P-aminosalicylic acid
P-aminosalicylic acid (PAS) can be considered as the last resource for treatment of MDR/RR-TB. It is often poorly tolerated and has a modest bacteriostatic activity. The drug is recommended in the WHO consolidated guidelines only for use in the treatment of MDR/RR-TB patients on longer regimens if bedaquiline, linezolid, clofazimine or delamanid are not used, or if better options to compose a regimen are not possible. There is no indication of cross-resistance of PAS to other anti-TB drugs (1). Use of PAS is limited owing to poor gastrointestinal tolerance.
Other medicines
Some medicines previously recommended as potential components of MDR-TB longer treatment regimens do not feature in Groups A–C.
High-dose isoniazid
High-dose isoniazid is not included in Groups A–C given the rarity of its use in longer regimens for adults. It is considered a relatively safe medicine, as shown recently in experience with its use at the 10 mg/kg dose, where only 0.5% of 1006 patients in a multicentric observational study of the shorter MDR-TB regimen reported Grade 3 or 4 neurotoxicity (117). Other evidence suggests that high-dose isoniazid may also be useful in the longer MDR-TB regimens. First, in the systematic review and IPD meta-analysis commissioned by WHO in 2015 to describe treatment outcomes in children with MDR-TB (which included 975 children in 18 countries), the use of high-dose isoniazid was associated with treatment success among children with confirmed MDR-TB (adjusted odds ratio [aOR] 5.9, 95% CI: 1.7–20.5, P=0.007) (118, 119). Second, in a randomized, double-blinded, placebo-controlled clinical trial among adults with MDR-TB, participants who received high-dose isoniazid (16–18 mg/kg) (added to kanamycin, levofloxacin, prothionamide, cycloserine and PAS) were significantly more likely to experience culture conversion at 6 months of treatment than those receiving placebo or standard-dose isoniazid (5 mg/kg) (73.8% versus 48.8% or 45.0%, respectively), with median time to culture conversion significantly reduced in the high-dose isoniazid arm (3.4 versus 6.6 or 6.4 months, respectively) (120). Third, a more recent early bactericidal activity study among patients with MDR-TB – in which the isoniazid resistance was mediated by isolated inhA mutations – demonstrated that doses of 10–15 mg/kg of isoniazid daily exhibited bactericidal activity similar to standard-dose isoniazid (5 mg/kg) given to patients with DS-TB (121). Strains with isolated katG or both katG and inhA mutations are unlikely to respond even to high-dose isoniazid, given the typically high isoniazid MICs in those strains. In the absence of information on isoniazid mutation patterns for an individual patient, knowledge of the prevalence of both mutations among locally circulating RR-TB strains (e.g. from DRS in the relevant epidemiological setting) may also inform decisions as to which treatment regimens would be most appropriate.
6.2.3 Duration of the regimen
The total length of a long treatment regimen is 18 to 20 months.
Three evidence-based recommendations guide the duration of the longer MDR-TB regimens:
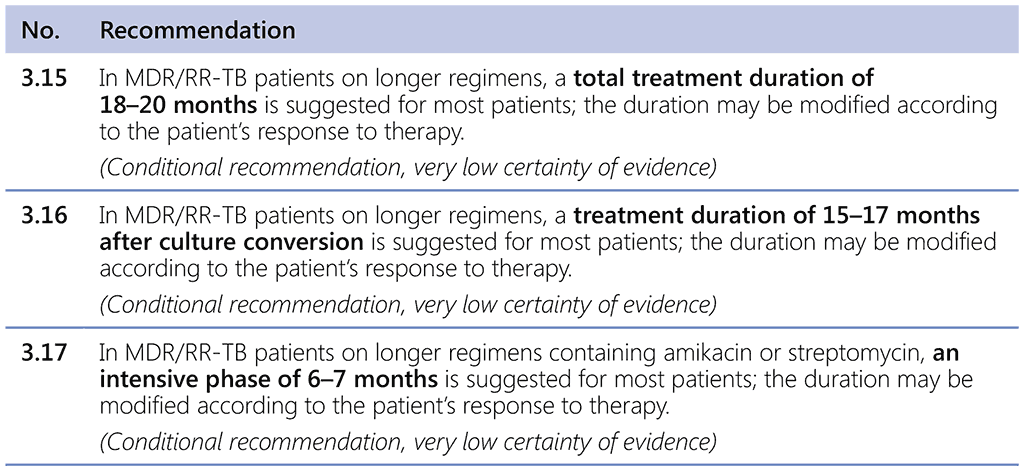
The all-oral longer MDR-TB regimens have no intensive phase. The duration of use of different medicines will depend on their clinical indication, patient tolerability (e.g. linezolid used for as long as no serious AE emerges) and individual treatment response (e.g. culture negativity), until completion of the expected total duration of treatment or time after culture conversion.
Although the total length of treatment is expected to be about 18–20 months in most patients, it may be modified based on the patient’s clinical situation and response to treatment.
The evidence assessed using the IPD²⁴ demonstrated that there was a marginally increased risk of treatment failure or relapse when the duration of MDR-TB treatment was 20–22 months (compared with 17.5–20.0 months), and 18–20 months was determined to be an optimal treatment duration to maximize treatment success (1). In practice, NTPs may choose a fixed duration (e.g. 18 months) for implementation purposes.
6.3 Key subgroups
6.3.1 People living with HIV
Currently, there are no specific changes in using longer regimens in PLHIV. However, there can be cumulative risk factors for clinical complications, toxicities and DDIs (Annex 1 and Annex 2); hence, these patients may need close follow-up and support.
6.3.2 Children
WHO recommendations on longer MDR/RR-TB regimens apply to children as well as adults. Currently, there is no age restriction on the use of bedaquiline, so children of all ages should receive it in longer regimens unless there is a specific contraindication. Most medicines in longer regimens have been part of MDR/RR-TB regimens for many years, in similar combinations, for both adults and children. Second-line TB medicines are also available in child-friendly formulations. The dosage for children is available in the Annex 4, including regular and dispersible medication. The duration of treatment using longer regimens in children depends on the site and severity of disease, and the extent of resistance. Children with non-severe disease can usually be treated for much less than 18 months. Children with extensive disease may require longer treatment durations, depending on clinical progress or site of the disease.
6.3.3 Pregnant and breastfeeding women
Dosing and safety data to support the optimal use of second-line TB medicines during pregnancy are generally sparse. There have been case reports and observational data reporting successful treatment and pregnancy outcomes among women who received treatment (including bedaquiline-containing regimens) for MDR/RR-TB during pregnancy and postpartum, but pregnant and breastfeeding women are usually excluded from clinical drug trials and early access programmes. Even less is known about the effects of MDR/RR-TB treatment on the infant in-utero and after birth; however, in general, the benefits (to both parent and child) of providing effective MDR/RR-TB treatment to the parent far outweigh the potential risks posed to the fetus in-utero or the breastfed infant.
Ethionamide is usually contraindicated in pregnancy because animal reproduction studies have shown an adverse effect on the fetus, and there are no adequate and well-controlled studies in humans. The adverse effects of linezolid may be exacerbated by the physiological effects of pregnancy, which lead to a relatively low Hb (due to the dilutional effect of increased blood volume) and a higher risk of peripheral neuropathies at treatment baseline compared with non-pregnant patients. Nevertheless, linezolid may be considered for pregnant and breastfeeding patients. More compelling evidence on the dosing and safety of specific anti-TB drugs among pregnant and breastfeeding women is needed to guide decision-making on the most appropriate regimen for treatment of MDR/RR-TB during pregnancy and postpartum. Amikacin and streptomycin are considered teratogenic and should be avoided during pregnancy. They should be considered only if there is no other option and the lives of the pregnant person and fetus are at risk.
Pregnant and breastfeeding women require considerable adherence support and monitoring of proper administration of MDR/RR-TB treatment, along with other chronic medications, to ensure successful treatment outcomes and minimal risk of TB transmission from mother to infant postpartum. Care providers must also pay particular attention to seamless continuity of care between antenatal and TB services; such services are rarely integrated in TB-endemic settings (106).
Considerations on the use of TB medication during pregnancy are given in Annex 1.
6.3.4 Patients with diabetes mellitus
Currently, there are no specific changes in patients with diabetes; however, such patients may present cumulative risk factors for clinical complications, toxicities and DDIs. Good glycaemic control is considered essential while on TB treatment because such control optimizes the chance of cure and limits complications. The concomitant use of metformin at high doses and linezolid may increase the risk of lactic acidosis. Also, the long-term use of linezolid, high doses of isoniazid and cycloserine in patients with diabetes can lead to an increased risk of peripheral neuritis. Baseline optic nerve or retinopathy or maculopathy may worsen after linezolid use; hence, eye evaluation is recommended before and during treatment. Regarding potential baseline renal damage, amikacin and streptomycin should be used with caution. Patients with DR-TB and diabetes may need close follow-up and support, with quick identification of DDIs and AEs.
6.3.5 Patients with extrapulmonary TB
The WHO recommendations on longer MDR-TB regimens also apply to patients with extrapulmonary disease. Adjustments may be required, depending on the specific location of disease. Treatment of MDR/RR-TB meningitis is best guided by DST of the infecting strain and by the ability of TB medicines to cross the blood–brain barrier. Group A FQ (e.g. levofloxacin, moxifloxacin and linezolid) have good penetration across the blood–brain barrier (i.e. the CNS), as do ethionamide (or prothionamide), cycloserine (or terizidone) and imipenem–cilastatin (122–124). Seizures may be more common in children with meningitis treated with imipenem, and meropenem is preferred for cases of TB meningitis and in children (125–127). High-dose isoniazid and pyrazinamide can also reach therapeutic levels in the CSF and may be useful if the strains are susceptible. Neither PAS nor ethambutol penetrate the CNS well and they should not be considered effective agents for MDR-TB meningitis. Amikacin and streptomycin only penetrate the CNS in the presence of meningeal inflammation. Data are sparse on the CNS penetration of clofazimine, bedaquiline or delamanid.
6.4 Implementation considerations and treatment in special situations
6.4.1 Extensive DR-TB disease
Extensive (or advanced) TB disease in adults is defined as the presence of bilateral cavitary disease or extensive parenchymal damage on chest radiography. In children aged below 15 years, extensive (or advanced) disease is usually defined by the presence of cavities or bilateral disease on chest radiography. This highlights the importance of chest radiography as part of the diagnostic work-up for patients, along with bacteriological tests. Patients with extensive disease tend to have a higher bacterial burden, especially in cases of parenchymal lung destruction (e.g. lobe collapse, fibrotic tracts or atelectasis), where drug concentration might be low due to decreased tissue perfusion. These patients tend to benefit from longer regimens to decrease the chances of relapse on shorter regimens. Patterns of lung destruction tend to present a higher risk of negative outcomes such as treatment failure and clinical complication (e.g. bacterial, fungal or mycobacterial superinfections, bronchiectasis and respiratory failure). Disability after cure is frequent. Close follow-up is needed during and after TB treatment.
6.4.2 Severe extrapulmonary TB
A longer treatment regimen may be more suitable in cases of severe extrapulmonary TB, owing to the high risk of negative outcomes including relapse. All such cases have in common the dispersion of Mtb through blood. Severe extrapulmonary TB is associated with lesions in multiple organs, potentially leading to multiorgan failure. This is more frequently suffered by patients with frank or relative immunosuppression (PLHIV, children, pregnant people, older people, people with cancer, those with solid organ transplants, those on immunosuppressive medication and people with uncontrolled diabetes mellitus). The potential reasons for immunosuppression should be addressed and all potential complications managed. Corticosteroids should be considered case by case, but are recommended in TB meningitis and pericardial TB to reduce complications and disability.
6.4.3 DR-TB in different patient groups
DR-TB meningitis and brain tuberculomas
When TB affects the CNS it leads to several additional problems. For example, the concentrations of some drugs in the CNS can be reduced owing to low penetration through the blood–brain barrier. Therefore, drugs need to be selected on the basis of both susceptibility and specific CNS penetration. Drugs with high CNS penetration should be used.
Information on each drug’s CNS penetration is given in Annex 1. Where options are limited, drug dosages can be increased to better reach the CNS, but with close monitoring of toxicity. Also, IV medication can be considered as the route of administration to optimize the drug concentration in blood while avoiding potential malabsorption problems. Patients with TB in the CNS may present with reduced consciousness and may require hospitalization, nutritional support (e.g. nasogastric tube and use of dispersible or IV medication) and, in advanced cases, intensive care. In all TB meningitis cases, the use of corticosteroids should be considered, to prevent disability and improve survival. Usually, when there is TB in the CNS, this is by haematogenous dissemination; therefore, it is important to search for the presence of TB in other organs such as lungs (e.g. bronchogenic or miliary TB), liver, spleen and bone marrow.
DR-TB in older patients
Patients with MDR/RR-TB who are aged 65 years and older are generally frailer and more vulnerable to the adverse effects of TB medications owing to the physiological changes of ageing (e.g. increase in QT interval, and baseline renal, eye or hearing damage). Also, they are more likely to present with other comorbidities (e.g. diabetes mellitus or hypertension) and therefore to be on other medications (i.e. to have a higher likelihood of polypharmacy), meaning there is a greater potential for additive drug toxicities and interactions. In addition, TB can be a consequence of a decline in the immune system due to age (immunosenescence), meaning that older patients may present with complicated forms of extrapulmonary TB.
DR-TB patients with renal failure
Patients with renal failure may be older, have diabetes or present with other comorbidities and use of multiple medications; thus, an in-depth evaluation is needed for each case. Patients with renal failure may present a baseline anaemia (possibly a clinical complication) that may be made worse by the use of linezolid or another myelotoxic drug. For many anti-TB drugs, dosage and administration may need to be adjusted according to levels of renal function. Annex 1 has detailed information on the use of each specific drug in renal failure.
DR-TB in patients with anaemia
Patients with TB often have anaemia, and treatment with an effective drug regimen may lead to improvement or resolution of the anaemia once the disease is properly treated. In the case of disseminated TB, Mtb itself may be suppressing bone marrow function. Malnutrition is also associated with anaemia, which often presents as low Hb, iron deficiency and low iron stores. Iron and multivitamins are recommended, but may interact with the absorption of important drugs such as FQ (requiring intake separated by >2 hours). In the case of severe anaemia, blood transfusion can be considered. Some of the drugs that are often used in patients with TB (e.g. linezolid, azidothymidine and co-trimoxazole) can also lead to bone marrow suppression and should be used with caution.
DR-TB in malnourished patients
Malnutrition is frequently found in children and adults with TB. Malnutrition can be a cause or a consequence of TB disease. A low BMI (<18 kg/m² , and especially <14 kg/m² ) is considered a risk factor for negative outcomes. Immune system function is decreased in malnourished patients; thus, more complicated extrapulmonary TB affecting critical organs may develop. In a patient with malnutrition, many other complications and superinfections can coexist, making clinical management much more complex; such patients also then require more medication, with potential DDIs. Malnourished patients may have poor tolerance for the daily intake of medication (owing to gastrointestinal issues), with frequent nausea, vomiting and diarrhoea. In addition, malnourished patients tend to present with malabsorption; thus, even if the intake of the medication is correct, the concentrations of anti-TB medication in blood can be suboptimal. Malnourished patients require close monitoring and a nutritional approach while on TB treatment; they may even benefit from IV administration of TB medication for short periods until there is improvement (either clinical or nutritional). Close monitoring of side-effects and an in-depth clinical evaluation is needed, to identify additive superinfections or comorbidities. Nutritional supplements could help malnourished patients to recover by strengthening their immune system and improving weight gain.
DR-TB in patients with hepatitis B or C
There are limited data on the use of the longer treatment regimen among people with viral hepatitis or undergoing treatment for hepatitis C. It may be prudent to monitor closely for DDIs and hepatotoxicity among this patient group.
DR-TB in patients with depression
Mental suffering and depression is common in DR-TB patients, because of, for example, symptomatic and life-threatening disease, side-effects, stigma and social exclusion, inability to work and family catastrophic costs. Some TB medications such as cycloserine (and to a lesser extent isoniazid and ethionamide) can trigger depression and suicidal ideation. These circumstances need to be seriously considered, especially in longer regimens, because depression and the social and emotional circumstances around it are often linked to difficulties in treatment adherence. Linezolid could potentially interact with all antidepressant drug families, increasing the risk of serotonergic syndrome (Annex 1 has more detailed information on linezolid DDIs). A balance between risk from TB and depression needs to be considered.
DR-TB in patients who present with alcohol or other substances abuse
Patients with DR-TB presenting with alcohol or other substances abuse is a situation that is often associated with the depression and social vulnerability that occurs particularly with TB in big cities. In addition to the negative emotional impact of DR-TB, anti-TB medication can have a negative effect on the patient. Cycloserine is associated with mood changes and potentially with craving and overconsumption of food, and methadone and psychiatric medication may interact with linezolid. A comprehensive patient-centred approach and harm reduction models that include psychosocial support are especially needed in these patients and had been shown to improve outcomes.
6.5 Treatment monitoring
Individuals prescribed the longer treatment regimen should be monitored to assess regimen effectiveness and safety, taking into account resistance patterns and challenging clinical conditions, while using less active and more toxic medicines. The WHO framework for aDSM needs to be applied to patients on any MDR-TB regimen, to ensure appropriate action and an acceptable level of monitoring for and prompt response to AEs – alongside monitoring for treatment outcomes, including early monitoring for treatment failure.
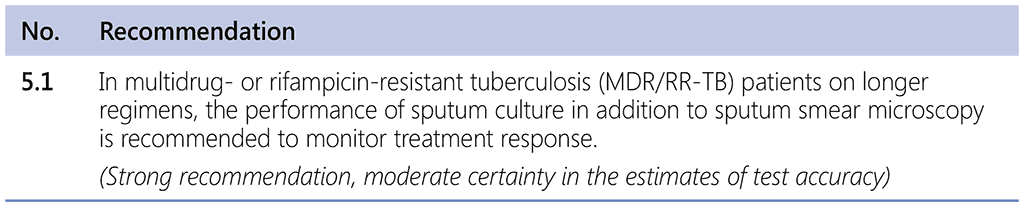
It is desirable for sputum culture to be repeated at monthly intervals.
Table 2.6.2. Summary algorithm for longer MDR-TB regimen composition in commonplace situations of resistance pattern or contraindication
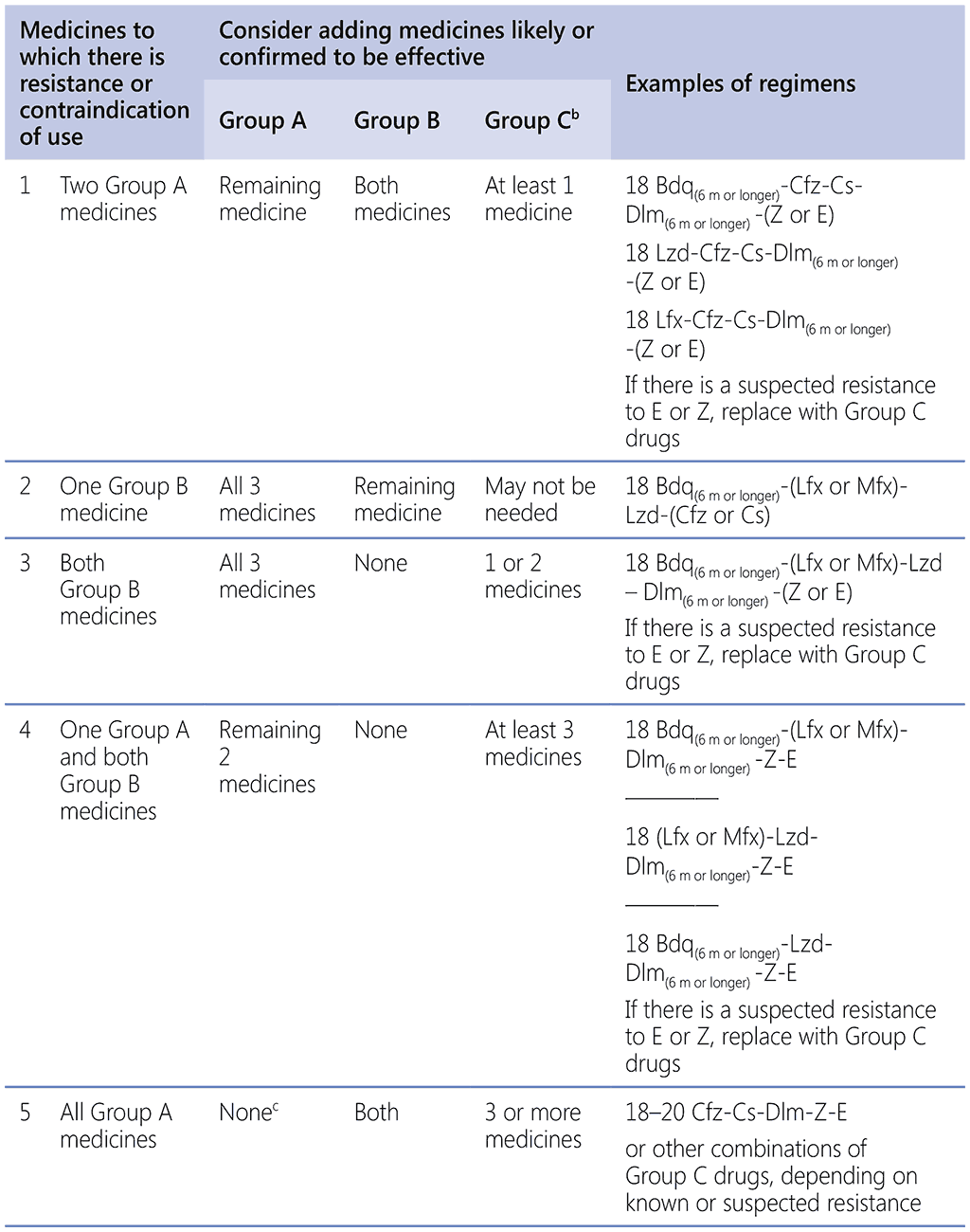
CB: clinical breakpoint; m: months; MDR-TB: multidrug-resistant TB; MDR/RR-TB: multidrug- or rifampicin-resistant TB; MIC: minimum inhibitory concentration; TB: tuberculosis; WHO: World Health Organization.
Drugs: Bdq: bedaquiline; Cfz: clofazimine; Cs: cycloserine; Dlm: delamanid; E: ethambutol; Lfx: levofloxacin; Lzd: linezolid; Mfx: moxifloxacin; Z: pyrazinamide.
a The situations shown are not exhaustive. Other factors may influence choice, such as patient risk for poor outcome or drug–drug interactions, clinician and patient preference and availability of a medicine. More medicines may be added than the recommended minimum if there is limited confidence in the effectiveness of regimen components, or if the patient was exposed in a setting where second-line TB drug resistance is frequent and longer MDR-TB regimens perform poorly despite good programmatic management of MDR/RR-TB. For MDR-TB with confirmed FQ resistance, no FQ is used and, if Group C agents are needed, the recommended WHO grouping will be followed based on benefit versus risk and individual circumstances.
b The choice and number of Group C medicines to include depends on the confidence in the effectiveness of medicines in this group and the other components of the regimen, thus:
• if 4 Group A and B agents are included and there is confidence in all of them, then Group C agents are not needed;
• if 3 Group A and B agents are included and there is confidence in all of them, then at least one Group C agent is added; and
• if 2 Group A and B agents are included and there is confidence in all of them, then at least three Group C agents are added.
c Moxifloxacin, a later-generation FQ, may still be effective at a high dose when the FQ MIC is below the CB. If the MIC is elevated, then FQ are not used, and additional Group C agents will be needed.
23 Imipenem–cilastatin and meropenem are administered with clavulanic acid, which is available only in formulations combined with amoxicillin. Amoxicillin–clavulanic acid is not counted as an additional effective TB agent, and should not be used without imipenem– cilastatin or meropenem.
24 Data used for analysis to support these recommendations were from patients who did not receive two or more Group A medicines. However, a small proportion of patients included in the analysis were on all-oral regimens, and in these patients the same optimal treatment duration was observed using identical parameters.

 Retour
Retour