Liens transversaux de livre pour PLATEFORME DE PARTAGE DES CONNAISSANCES SUR LA TUBERCULOSE DE L'OMS
DS-TB is largely curable with treatment that is affordable and widely accessible. If a TB treatment regimen is not administered correctly, it may fail to deliver a relapse-free cure, thus increasing transmission and accelerating the emergence of drug resistance. Monitoring the effectiveness of TB treatment is thus critically important in both clinical practice and surveillance, to maximize the quality of individual patient care and the effectiveness of public health action. Hence, standardized TB treatment outcome definitions have been a feature of WHO policies and national TB surveillance systems for many years as a cornerstone of effective TB strategies. This standardization has allowed the monitoring of TB treatment outcomes over time at national and global levels.
Standardized treatment outcome definitions for DS-TB have been in widespread use for more than 3 decades, and outcome definitions for DR-TB were first proposed in 2005 (96). The development of DR-TB treatment outcome definitions was based on the outcome definitions for DS-TB in use at the time. The DR-TB treatment outcome definitions were adopted by WHO soon after and remained largely unchanged until 2013, when WHO updated its TB definitions and reporting framework (97). As treatment regimens for DR-TB have significantly changed in composition and duration, an update of the treatment outcome definitions and monitoring parameters was necessary.
10.1 Treatment outcome definitions
In November 2020, the WHO Global TB Programme (WHO/GTB) convened an online consultation and released new definitions of TB treatment outcomes, which were the same for DS-TB and DR-TB (98–100).
The principles guiding the update of the definitions were as follows:
- applicability to treatment regimens of different duration;
- a lessening of the traditional division between the intensive and continuation phases;
- identification of appropriate criteria for bacteriological conversion (or reversion) in relation to the definitions of “treatment failed”, “cured” and “treatment completed” that are grounded in knowledge from microbiology;
- consideration of the use of appropriate diagnostics for treatment monitoring;
- setting of clear parameters for defining treatment failure, based on reliable evidence of non-response or other reasons that lead to a decision to change or stop treatment; and
- aiming for practical clinical and programmatic monitoring, and feasible implementation.
A new optional definition, “sustained treatment success”, was also proposed for use in operational research only. Post-treatment follow-up may be useful, when or if it is feasible, for patients suffering from post-treatment sequelae, for example (101).
The new treatment outcome definitions are summarized in Table 1.10.1.
The 2020 treatment outcome definitions allow all patients with either DS-TB or DR-TB to have a treatment outcome assigned when completing treatment (cure or treatment success) or when unfavourable events occur (e.g. loss to follow-up [LTFU], failure or death).
Although the definitions of treatment outcomes have been harmonized, minor differences remain between those for DS-TB and DR-TB (e.g. treatment monitoring by sputum culture for DR-TB and by sputum smear microscopy for DS-TB).
Despite some distinct treatment phases remaining in current regimens, the overall trend is towards monophasic regimens. Thus, it is best to avoid linking definitions to treatment phases; hence, the time thresholds for declaring cure or treatment failure have been revised.
Although the role of new bacteriological tests was considered, treatment monitoring will continue to rely on the available tools (i.e. sputum culture for DR-TB and sputum microscopy for DS-TB), despite their limitations.
10.2 Considerations for implementation
It is both important and feasible for NTPs to ascertain cure at the end of treatment. The notion of relapse-free cure or sustained treatment success after the end of treatment is critical; however, it is beyond the means of routine programmatic monitoring and is feasible only under operational research conditions (e.g. in special cohorts, in patients undergoing rehabilitation and during follow-up for post-TB lung disease). For this reason, the specific operational definition “sustained treatment success” was proposed (Table 1.10.1), with the possibility of assessing numbers of patients alive and free of TB at 6 months (for DS-TB and DR-TB) and at 12 months (for DR-TB only) after successful TB treatment.
Table 1.10.1. New definitions of TB treatment outcomes for both DS-TB and DR-TB
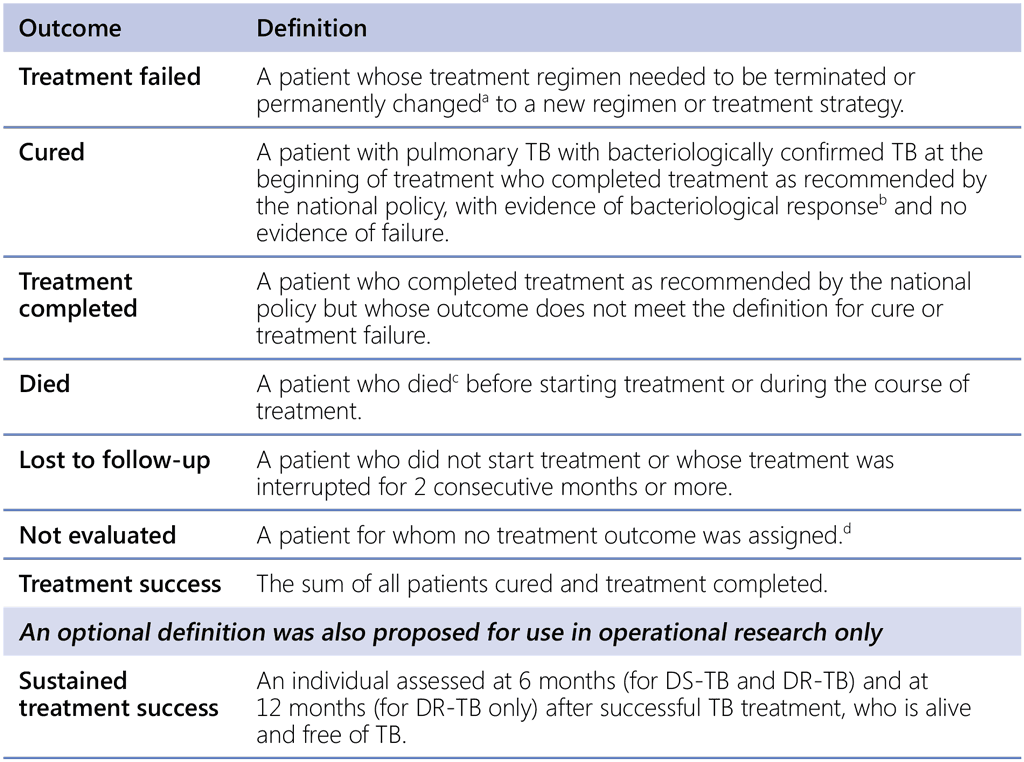
DR-TB: drug-resistant TB; DS-TB: drug-susceptible TB; TB: tuberculosis.
a Reasons for the change include:
• no clinical response or no bacteriological response, or both (see note ‘b’);
• adverse drug reactions; or
• evidence of additional drug-resistance to medicines in the regimen.
b “Bacteriological response” refers to bacteriological conversion with no reversion:
• “bacteriological conversion” describes a situation in a patient with bacteriologically confirmed TB where at least two consecutive cultures (for DR-TB and DS-TB) or smears (for DS-TB only) taken on different occasions at least 7 days apart are negative; and
• “bacteriological reversion” describes a situation where at least two consecutive cultures (for DR-TB and DS-TB) or smears (for DS-TB only) taken on different occasions at least 7 days apart are positive either after the bacteriological conversion or in patients without bacteriological confirmation of TB.
c Patient died for any reason.
d This includes cases “transferred out” to another treatment unit and whose treatment outcome is unknown; however, it excludes those lost to follow-up.

 Retour
Retour