Liens transversaux de livre pour 1266
8.1 Surgery in the treatment of MDR/XDR-TB
Surgery has been employed in the treatment of TB since before the advent of chemotherapy. With the challenging prospect that more cases of MDR/XDR-TB are virtually untreatable with all available drugs or risk having serious sequelae, there has been re-evaluation of the role of pulmonary surgery as a way to reduce the amount of lung tissue with intractable pathology and to reduce the bacterial load. Large case series have reported that resection surgery may be safe and an effective adjunct when skilled thoracic surgeons and excellent postoperative care are available (140, 141).
The WHO consolidated guidelines include a conditional recommendation for elective partial lung resection (lobectomy or wedge resection) as an adjunct to the chemotherapy of MDR/RR-TB patients with resistance to additional medicines. The recommendation does not apply to radical pneumonectomy, which had no statistically significant effect (140). The recommendation was based on evidence from an IPD meta-analysis to evaluate the effectiveness of different forms of elective surgery as an adjunct to combination medical therapy for MDR-TB (140), and a systematic review and study-level meta-analysis (142).
The relative benefits of surgery are expected to depend substantially on the population subgroups that are targeted. The reviews for the guideline update in 2016 (8) could not provide a refined differentiation of the type of patient who would be best suited to an intervention, or the type of intervention that would carry the most benefit. The effect is expected to be moderate in the average patient considered appropriate for surgery. The odds of success for patients with MDR/RR-TB and resistance to FQ and injectable agents were significantly lower when they underwent surgery compared with other patients (aOR: 0.4, 95% CI: 0.2–0.9) (140). This finding is likely to be biased, given that patients who underwent surgery would have had other factors predisposing them to poor outcomes – factors that could not be adjusted for. Programmes with limited access to surgery may target patients who remain sputum smear positive, who have resistance to many drugs and who have localized pulmonary disease. Computerized tomography, pulmonary function testing and quantitative lung perfusion or ventilation may have a role in the preoperative work-up.
Resection surgery should be timed to give the patient the best possible chance of cure with the least risk of harm. Thus, the timing of surgery may be earlier in the course of the disease when the patient’s risk of morbidity and mortality are lower (e.g. when the disease is still localized to one lung or one lung lobe). Generally, at least 2 months of therapy should be given before resection surgery, to decrease the bacterial infection in the surrounding lung tissue. Prognosis appears to be better when partial lung resection is performed after culture conversion. Even with successful resection, the total duration of treatment and the duration of treatment after culture conversion should be guided by the recommendations in Sections 4, 5, 6 and 7.
Partial lung resection for patients with MDR/RR-TB is only to be considered when good surgical facilities, staffed by trained and experienced surgeons, are available. Many programmes will have limited access to surgical interventions. In programmes with suboptimal surgical facilities and with no trained thoracic surgeons, resection surgery may increase morbidity or mortality. Specialized surgical facilities should include stringent infection control measures (given that infectious material and aerosols are generated in large quantities during surgery), mechanical ventilation and postoperative pulmonary hygiene manoeuvres. After resection, direct laboratory testing of the resection material (lung lesion) will be useful. If the results of laboratory testing differ between the resected material and other clinical specimens, the treating clinician may need to adjust treatment based on the results obtained from the resected material or other clinical specimens.
There are still many uncertainties about the role of surgery in MDR-TB treatment. All data available for the 2016 recommendations were from observational data from case series, which may be biased. For instance, it is likely that in choosing patients to be operated on there would have been systematic exclusion of patients deemed unfit for surgery and anaesthesia, such as older patients and those who were very sick with comorbidities (e.g. no patient with HIV in the dataset had undergone surgery) or extensive disease. There were not enough data on AEs, surgical complications or long-term sequelae – some of which may be fatal – to allow for a meaningful analysis. Conversely, the effectiveness of surgery may have been underplayed in the analysis because of the lack of a suitable control group.
8.2 Use of corticosteroids
Corticosteroids have been used to support the treatment of serious and severe consequences of TB, such as miliary TB, respiratory insufficiency, CNS involvement and pericarditis.
The WHO Guidelines for treatment of drug-susceptible TB and patient care, 2017 update made the following recommendations (2, 143):
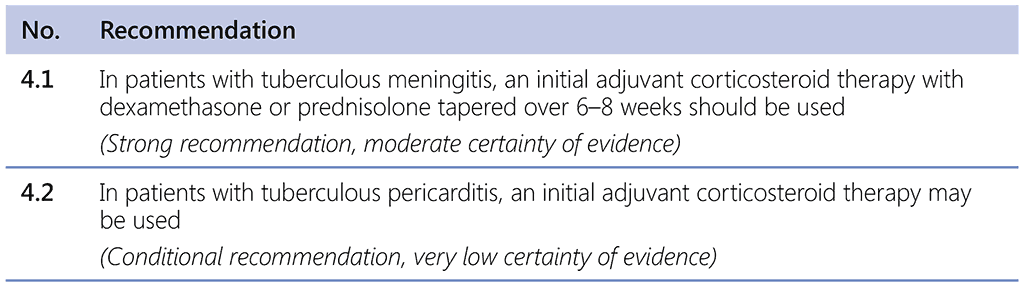
The recommendations are limited to these two forms of extrapulmonary TB. In patients with TB meningitis, evidence from RCTs (144–148) showed lower rates of death, severe disability and relapse when patients received steroids with TB treatment. The mortality benefit increased with increasing severity of TB meningitis. AEs and severe AEs, including severe hepatitis, were lower in patients receiving steroids. In patients with TB pericarditis, studies showed a benefit to steroid treatment in relation to death, constrictive pericarditis and treatment adherence (149–156).
Although the evidence and the recommendations primarily relate to non-MDR-TB, these recommendations could also apply to patients with MDR/RR-TB, on the condition that the patient is still receiving the TB treatment regimen. Corticosteroids are immunosuppressive and therefore can weaken the body’s response to fight TB; hence, they should only be used if clearly indicated and if the patient is on an adequate effective regimen. If corticosteroids are used in an inadequate regimen, this could accelerate the patient’s deterioration. Oral treatment can be given, but when a more immediate response is needed, injectable corticosteroids are often used initially.
8.3 Treatment of MDR/RR-TB patients with HIV
With regard to HIV infection, a specific recommendation was made in 2011 on the use of ART in all patients with HIV and DR-TB (80, 128):

Delaying ART increases the risk of dying among TB patients living with HIV; therefore, ART should be started in all TB patients living with HIV, regardless of their CD4 cell count. The therapy should be initiated as soon as possible within the first 8 weeks of TB treatment, or within the first 2 weeks in patients with profound immunosuppression (e.g. CD4 counts <50 cells/mm³ ). In children with HIV and active TB, ART should be initiated as soon as possible and within 8 weeks following the initiation of anti-TB treatment, regardless of the CD4 cell count and clinical stage (157).
There may be a potential for overlapping, additive toxicities or DDIs between some antiretroviral medicines and the injectable agents, moxifloxacin and clofazimine; however, there are usually no grounds to warrant modifications of the MDR-TB or the ART regimens. While no interactions are anticipated for the preferred first-line ARV, dolutegravir, it is not recommended to use bedaquiline and efavirenz in combination (Annex 2). Annex 1 provides information on individual medicines used to treat MDR/RR-TB and their drug interactions. In addition, information on HIV drug interactions is available on the HIV drug interactions webpage (158). Antiretroviral treatment regimens should be initiated early, in accordance with WHO recommendations (15, 80). Close monitoring for response and toxicity is advised for patients on both TB and HIV treatment. Other comorbidities (e.g. diabetes and mental health disorders) should be managed accordingly (15).
8.4 Treatment of MDR/RR-TB patients coinfected with HCV
This section refers to a treatment recommendation for people with confirmed MDR/RR-TB and infection with hepatitis C virus (HCV). The new recommendation in the updated 2024 guideline states:

Remarks
- This recommendation applies to people with confirmed MDR/RR-TB and HCV.
- Treatment initiation should take into account potential DDI and other comorbidities.
8.4.1 Eligibility
Individuals with confirmed HCV and MDR/RR-TB can receive both treatments. The composition and duration should follow the current recommendation (159).
8.4.2 Implementation considerations
Co-administration of MDR-TB and HCV treatments
Initiating co-administered treatment for HCV and MDR/RR-TB requires careful consideration of potential DDIs and other comorbidities. This is important because, globally, MDR/RR-TB treatment success rates remain low (only 63% in 2022). Coinfection with HCV further complicates treatment, because individuals are at increased risk of liver damage (hepatotoxicity) due to certain anti-TB medications (160).
There is an overlap in the risk factors for both HCV and TB. Chronic viral hepatitis B or C can further negatively impact TB treatment by increasing the risk of drug-induced hepatotoxicity (161). Fortunately, the development of short-course, oral DAAs has revolutionized HCV treatment, achieving cure rates exceeding 90%, with minimal side-effects (161, 162).
A recent systematic review identified limited direct evidence on co-administration; however, expert analysis suggests potential benefits. Co-administering treatment for MDR-TB and HCV may improve MDR-TB treatment success, reduce treatment failures and decrease LTFU (163). Although data on HCV treatment outcomes from co-administration are scarce, the potential advantages of MDR-TB treatment outweigh the uncertainties. Therefore, co-administration is conditionally recommended for MDR/RR-TB patients with confirmed HCV coinfection and delaying HCV treatment is discouraged (163). The decision to administer both treatment regimens should be informed by knowledge of potential DDIs and patient preferences. Importantly, if HCV treatment is not available, this should not impede the initiation of MDR-TB treatment.
It is important to acknowledge limitations in the available data, particularly for pregnant women, PLHIV and children. Caution is advised when applying the findings to these groups. Currently, there are no official recommendations for HCV treatment in pregnancy; however, this is an evolving area, and emerging evidence will guide future development of treatment recommendations. Clinicians should make co-administration decisions based on individual patient factors, including a thorough understanding of potential DDIs and existing comorbidities. Transparency regarding the current evidence limitations is crucial when discussing treatment options with patients.
Supporting patient adherence is vital throughout treatment, particularly considering the potential for shorter regimens. Health care providers should prioritize strategies aimed at enhancing adherence and empowering patients to successfully complete both treatment courses.
In summary, co-administration of MDR-TB and HCV treatment is the suggested approach for individuals diagnosed with MDR/RR-TB and HCV. Although there are some data limitations, the potential benefits of improving MDR-TB treatment outcomes outweigh the risks. Effective implementation should prioritize patient support and clear communication.
Access to treatment for HCV and MDR-TB
The coordination of HCV and MDR-TB treatments necessitates access to both groups of medicines, with initiation of MDR-TB treatment not delayed if HCV treatment is not available. Programmes must also have access to reliable DST for MDR-TB medicines and bacteriological tests, as well as the ability to monitor the virological response for HCV.
8.4.3 Monitoring treatment response and outcome assignment
Close monitoring of the treatment response is critical throughout the entire duration of therapy.
HCV treatment progress is tracked through accurate viral load measurement; a sustained virological response at 12 weeks post-treatment indicates a successful cure. Given the potential impact of HCV treatment on liver health, regular LFT are essential to detect any signs of liver damage during treatment.
In MDR/RR-TB, the response to treatment is evaluated through bacteriological monitoring using regular sputum smear microscopy and culture, ideally monthly (17, 164). Regular clinical monitoring ensures timely adjustments of treatment and informed decision-making.
The treatment outcome definitions and reporting framework for patients receiving both HCV and MDR-TB treatments align with those for all DS-TB and DR-TB regimens (see Section 10, Chapter 1). Beyond treatment completion, follow-up evaluations are critical to monitor for potential relapse (Section 9.9).
8.4.4 Monitoring safety
Treatment monitoring schedules should encompass relevant clinical and laboratory parameters, to promptly detect, manage and prevent common and serious AEs. Although no significant risks were identified in the available data, the active drug-safety monitoring (aDSM) framework should be used for promptly detecting, managing and reporting any suspected or confirmed drug toxicities.
There is a paucity of data on DDIs between HCV treatments that use DAAs and MDR-TB medicines. Based on the limited published data available, current evidence suggests minimal interactions; however, caution is still advised.
It is notable that bedaquiline, a key component of most MDR-TB regimens, may increase the risk of liver toxicity, particularly when co-administered with some HCV treatments. Additionally, some MDR-TB drugs (e.g. ethionamide/prothionamide and clofazimine) may interact with specific DAAs (e.g. daclatasvir) by affecting how the body processes them, although this has not been definitively proven (72).
To address the paucity of published data on DDIs between anti-TB medications and HCV DAAs, health care professionals rely primarily on two key information sources:
- drug package inserts: these provide known DDIs for each medication; rifampin was the only anti-TB drug with established DDI studies, so the results of these studies were extrapolated to estimate potential DDIs for other anti-TB and HCV DAA combinations; and
- the University of Liverpool HEP drug interaction tool (165): this online resource aided in further evaluation and confirmation of potential DDIs; however, some anti-TB drugs (kanamycin, cycloserine, clofazimine, ethionamide and para-aminosalicylic acid) were not included in this database (162).
Using these resources, health care providers can assess potential DDIs by considering various HCV regimens and their predicted interactions with individual TB drugs used in the treatment of MDR-TB. This approach facilitates a more comprehensive understanding of possible DDIs, thereby informing treatment decisions and enhancing patient safety. As research in this area continues to evolve, it is crucial to approach concomitant treatment of TB and HCV with caution. Health care providers should remain vigilant and use available resources and emerging evidence to guide clinical decision-making, ensuring the best possible outcomes for patients with both conditions.
Potential DDIs were evaluated considering different HCV regimens and their predicted interaction with individualized TB drugs used for MDR-TB, as shown in Table 2.8.1.
Table 2.8.1. DDIs between MDR/RR-TB and HCV drugs
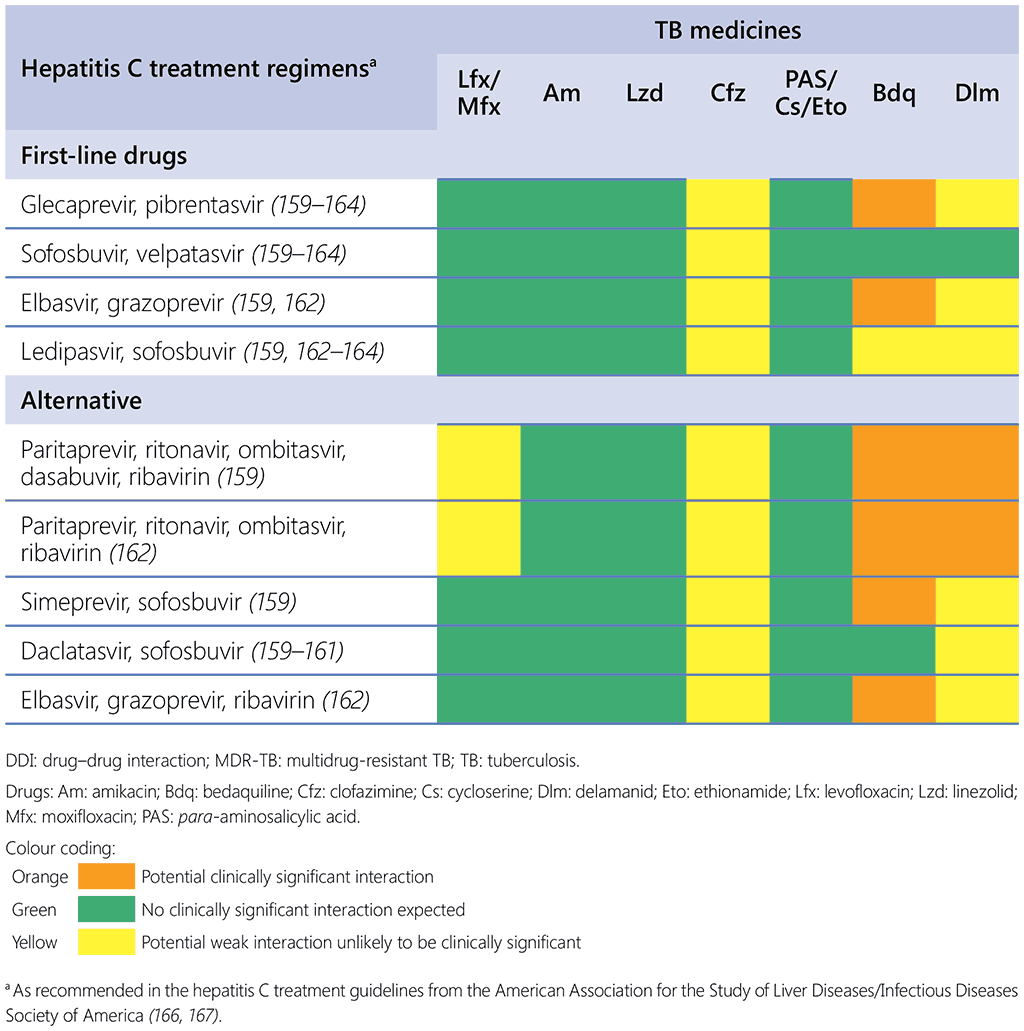
No TB medicines are advised for dosage adjustments in individuals with preexisting liver conditions (Table 2.8.2); nevertheless, it is crucial to conduct vigilant and regular monitoring of liver function, particularly for those with unstable or advanced liver disease, because certain studies indicate that such individuals may face an increased risk of drug-related liver damage (168). Given that many TB medications are metabolized in the liver, it is important to consider the potential influence of liver disease severity on the pharmacokinetics of anti-TB drugs (169).
Table 2.8.2. Characteristics of anti-TB drugs related to liver disease
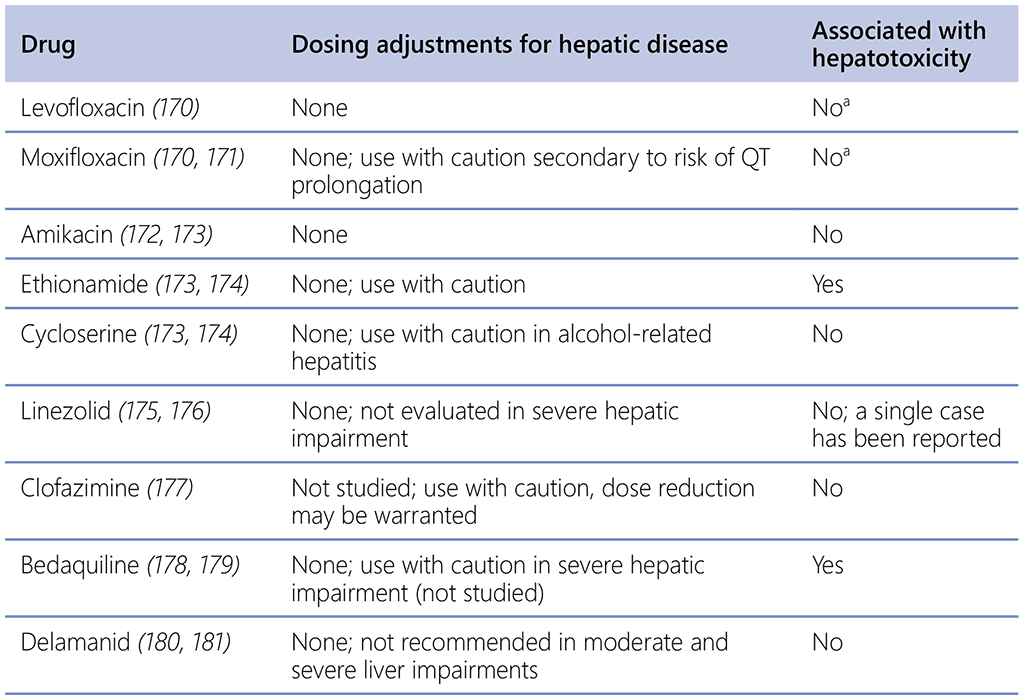
TB: tuberculosis.
a Although levofloxacin and moxifloxacin were associated with increased risk of acute liver injury compared to clarithromycin , they are generally not considered hepatotoxic drugs.
Given these potential interactions and characteristics of TB drugs related to liver disease, consulting with a specialist is essential. A specialist can assess individual patient factors and recommend the optimal treatment plan that minimizes DDIs and maximizes treatment success for both HCV and MDR-TB (162).

 Retour
Retour