Liens transversaux de livre pour 1266
2.1 TB diagnostics and DST
Innovative rapid molecular tests to diagnose both pulmonary and extrapulmonary TB in all populations are strongly recommended over sputum smear microscopy and culture methods, because rapid tests can provide same day results (3). Some of the innovative tests also provide drug susceptibility results for rifampicin (R), isoniazid (H) and fluoroquinolones (FQ), allowing rapid confirmation of diagnosis, and timely and effective treatment allocation. Based on the data from global TB drug-resistance surveillance, rifampicin-resistant TB (RR-TB) is rare when people are first diagnosed with TB, but it is reaching alarming proportions in some countries. Globally, the burden of multidrug-resistant TB (MDR-TB) or RR-TB (MDR/RR-TB) is stable (4). For more than 10 years, the best estimate of the proportion of people diagnosed with TB for the first time who had MDR/RR-TB has remained at about 3–4%, and the best estimate among those previously treated for TB has remained at about 18–21% (3). The highest proportions (>50% in previously treated cases) are found in countries in eastern Europe and Central Asia (4).
Resistance to FQ in new TB patients without resistance to rifampicin is rare in most of the countries with available data (1.0–1.2%), although some countries show higher proportions (3.4–11.2%) (5–9). Isoniazid-resistant and rifampicin-susceptible TB is the most prevalent form of drug resistance worldwide (besides streptomycin resistance), with estimates rising to 7% among those newly diagnosed and 8–11% among those previously treated (4, 10). Isoniazid-resistant TB is associated with a higher risk of acquiring further drug resistance and evolving towards MDR-TB, which is defined by resistance to both isoniazid and rifampicin (11–13).
These data reiterate the importance of full transition from sputum smear microscopy to the widespread use of rapid diagnostic tests, especially in those with recurrent TB. Regular country reports to WHO and several surveys clearly demonstrate that the policy of using rapid molecular TB diagnostic tests has been widely adopted in countries with a significant burden of TB. However, the use of rapid TB testing has yet to surpass the use of smear microscopy (14–16).
With the range of available anti-TB medicines and treatment regimens, drug susceptibility testing (DST) for key drugs is crucial for the choice of an appropriate treatment strategy. For medicines with high potency against Mycobacterium tuberculosis – rifampicin, moxifloxacin (M) and isoniazid – rapid tests for drug susceptibility are now available and evidence-based recommendations for their use are given in relevant WHO documents (3, 17).
Resistance to rifampicin renders all of the available regimens for DS-TB ineffective; if rifampicin is used, it may cause both treatment failure and development of additional resistance to other drugs in the regimen. This finding means that the treatment strategy for DR-TB needs to be changed. Among new TB patients without resistance to rifampicin, resistance to FQ is very low; this allows a general strategy of starting these patients on DS-TB regimens (including the 4-month regimen with moxifloxacin described in Section 4), without obligatory DST for FQ. This general strategy should be regularly revisited and updated in response to the drug-surveillance data of the country or specific setting, to prevent potential misuse of moxifloxacin (an important medicine for treatment of DR-TB) and increased antimicrobial resistance. Resistance to isoniazid leads to decreased efficacy of the 6-month regimen (described in Section 3) and requires use of the specific regimens that include FQ. The effect of isoniazid monoresistance on the efficacy of the 4-month regimen with rifapentine (P) and moxifloxacin has not been studied; however, the efficacy of the 4-month regimen for children (2HRZ(E)/2HR, described in Section 5) is expected to be affected.
In summary, in settings where rapid, molecular-based DST is available, the results should guide the choice of regimen. Although universal DST is the goal, priority should be given to testing patients undergoing retreatment at, or before, the start of that retreatment – at least for isoniazid and rifampicin resistance. Whenever rifampicin resistance is confirmed, testing for resistance to FQ will be important in the design of an effective treatment regimen.
In settings where rapid DST results are not routinely available to guide the management of individual patients, the approach to treatment selection can be guided by clinical judgement and consideration of the epidemiology of TB and its drug-resistant forms in the specific setting. In TB patients whose treatment has failed or in other patient groups with a high likelihood of MDR/RR-TB, the clinician’s decision may lean towards an empirical MDR-TB regimen (17).
2.2 Care and support during TB treatment
All treatment delivered should align with WHO-recommended standards, including patient-centred care and support, informed consent where necessary, principles of good clinical practice, and regular patient monitoring to assess regimen effectiveness and patient safety (Chapter 3). Clinical monitoring of people on treatment is important, and this handbook includes information on both treatment monitoring and the usefulness of post-treatment follow-up for special cases (e.g. long-term complications of TB or TB sequelae).
2.3 Options in treatment of DS-TB
In patients with presumptive or confirmed DS-TB, there are several regimens that can be used based on current WHO policy. The 6-month regimen has become the standard of care all over the world but efforts have been made to develop effective shorter regimens to treat DS-TB. Several trials were designed to assess whether a shorter treatment regimen can remain highly effective and raise no additional safety concerns. Based on the results of recent randomized controlled trials (RCTs), WHO has recommended two different 4-month regimens: one based on the Study 31 trial⁵ and one based on the SHINE trial⁶ (1).
Current recommendations cover regimens that differ in duration, composition and dosing of drug components. In addition, the eligible populations (depending on the available evidence) differ in terms of age and TB disease severity. The three recommended regimens are as follows:
- The 6-month regimen (2HRZ (E)/4 HR, described in Section 3) comprises 2 months of isoniazid, rifampicin, pyrazinamide (Z) and ethambutol (E), followed by 4 months of isoniazid and rifampicin. This regimen is recommended in all patient populations. In children (usually defined as being aged <10 years), the inclusion of ethambutol in the first 2 months of treatment is recommended in settings with a high prevalence of HIV,⁷ in settings with isoniazid resistance or in children living with HIV (CLHIV), but can otherwise be omitted, resulting in the 2HRZ/4HR regimen (19).
- The 4-month regimen HPMZ (described in Section 4) comprises 2 months of isoniazid, rifapentine, moxifloxacin and pyrazinamide, followed by 2 months of rifapentine, isoniazid and moxifloxacin. This regimen is recommended for all those aged above 12 years, whatever the severity of TB disease.
- The 4-month regimen HRZ(E) (described in Section 5) comprises 2 months of isoniazid, rifampicin and pyrazinamide, with or without ethambutol, followed by isoniazid and rifampicin for 2 months for those aged between 3 months and 16 years, with non-severe pulmonary or peripheral lymph node TB. The use of ethambutol in the first 2 months of treatment is recommended in settings with a high prevalence of HIV, in settings with isoniazid resistance⁸ or in children and adolescents living with HIV.
Choice criteria for regimens in different age groups are summarized in Table 1.2.1.
Table 1.2.1. Guide for regimen selection for DS-TB
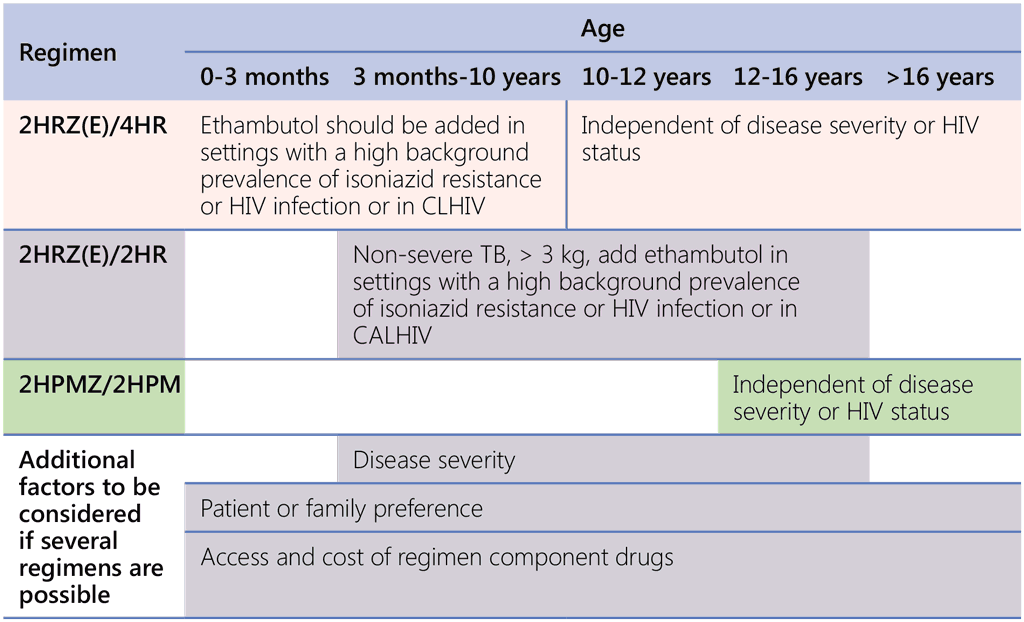
CALHIV: children and adolescents living with HIV; CLHIV: children living with HIV; DS-TB: drug-susceptible TB; HIV: human immunodeficiency virus; TB: tuberculosis.
Note: all the regimens envisage daily dosing of all medicines.
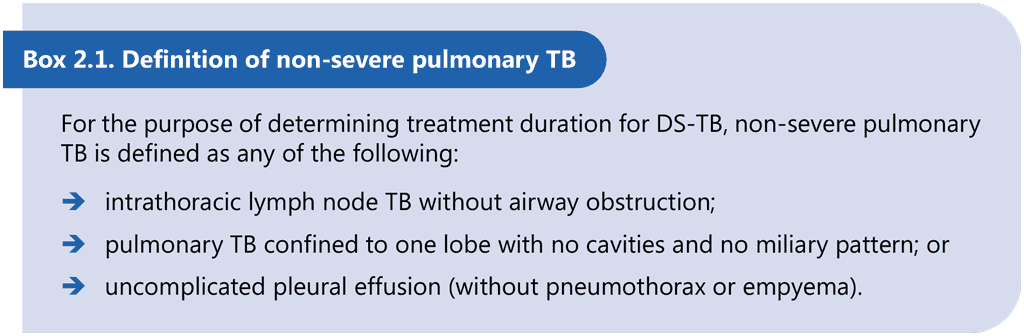
As shown in Table 1.2.1, the age ranges presented in the current recommendations are as follows: from 3 months to 16 years for one 4-month regimen (for 2HRZ(E)/2HR regimen), 12 years and above for the other 4-month regimen (for 2HPMZ/2HPM regimen) and any age for the 6-month regimen (2HRZ(E)/4HR). There is some overlap between age groups; for example, several regimen options are available for the age range 3 months to 12 years (i.e. 2HRZ(E)/2HR and 2HRZ(E)/4HR) and for the age range 12–16 years (i.e. 2HRZ(E)/4HR, 2HPMZ/2HPM and 2HRZ(E)/2HR). The choice of regimen may also be influenced by clinical factors (e.g. severity of the disease, hepatic or renal failure, uncontrolled diabetes and HIV status), contextual factors (e.g. HIV or prevalence of isoniazid resistance) and access factors (e.g. availability of rifapentine and moxifloxacin, and cost of the regimen). For example, severity of TB disease (based on the definition of non-severe TB presented in Box 2.1) will determine the choice of the regimen for those aged between 3 months and 16 years.
All patients weighing more than 3 kg and aged between 3 months and 16 years, with non-severe TB based on the definition presented in Box 2.1, should be treated with the 4-month regimen 2HRZ(E)/2HR, with or without ethambutol. It is preferred that CLHIV who receive the 4-month regimen receive that regimen with ethambutol for the first 2 months of treatment, irrespective of the background prevalence of HIV. In addition, it is strongly recommended that ethambutol be added to the 4-month regimen for the first 2 months in settings with a high background prevalence of isoniazid resistance or HIV infection.
The 6-month regimen comprising 2HRZ(E)/4HR can be used in all patients in all age groups, independent of the disease severity and HIV status. However, in patients aged under 10 years, ethambutol can also be omitted in patients who are HIV-negative or in settings with a low prevalence of HIV and isoniazid resistance.
In patients with DS-TB aged 12 years or more, another possible treatment option is the regimen comprising 2HPMZ/2HPM. This regimen can be used in patients with both severe and non-severe forms of the disease, and in people living with HIV (PLHIV). Access, the pill burden and the cost of the HPMZ regimen may present barriers for implementation until rifapentine becomes more widely and readily available at costs comparable with rifampicin, and the fixed-dose combination (FDC) tablet is developed and becomes available. In some cases, patient and family preference may also guide the choice of the regimen if several regimen options apply to the patient once all other factors have been considered.
In summary, for treatment of DS-TB, WHO recommends either a 6-month regimen or, in specific subgroups of patients, either of two new 4-month regimens (as presented in Table 1.2.1).
In patients who require TB retreatment, rapid DST should be performed to guide the regimen approach; that is, to determine whether the treatment should be for DS-TB or DR-TB. In 2017, the WHO Guideline Development Group (GDG) agreed to abolish the former Category II standard 8-month regimen (2HRZES/1HRZE/5HRE), which comprised an intensive phase of 3 months (2 months of isoniazid, rifampicin, pyrazinamide, ethambutol and streptomycin followed by 1 month without streptomycin), followed by a continuation phase of 5 months with isoniazid, rifampicin and ethambutol. The GDG put forward a good practice statement that this 8-month regimen should no longer be prescribed, and that DST should be conducted to inform the choice of the treatment regimen.
The three current regimen formulations and their durations are summarized below.
6-month regimen
New patients with pulmonary TB should receive a regimen containing rifampicin for 6 months. This 6-month treatment regimen, 2HRZ(E)/4HR, comprises isoniazid, rifampicin, pyrazinamide and ethambutol for 2 months followed by isoniazid and rifampicin for 4 months.
4-month regimens
Patients aged 12 years or more with pulmonary DS-TB may receive the 4-month regimen 2HPMZ/2HPM, which comprises rifapentine, isoniazid, pyrazinamide and moxifloxacin (2 months of isoniazid, rifapentine, moxifloxacin and pyrazinamide, followed by 2 months of isoniazid, rifapentine and moxifloxacin).
Children and adolescents aged between 3 months and 16 years with non-severe DS-TB should receive the 4-month regimen 2HRZ(E)/2HR, which comprises isoniazid, rifampicin and pyrazinamide, with or without ethambutol, for 2 months followed by isoniazid and rifampicin for 2 months.
Severe forms of TB such as tuberculous meningitis and osteoarticular TB may require additional clinical evaluation and judgement, and longer treatment regimens. Daily or weekly pyridoxine supplementation is suggested when giving isoniazid to patients with such forms of TB.
5 Study 31 is a Phase III trial: TBTC Study 31/ACTG A5349 (1), also referred to as S31/A5349.
6 SHINE is the Shorter Treatment for Minimal Tuberculosis in Children trial, a large Phase III trial to evaluate duration of TB treatment in children with non-severe DS-TB.
7 Defined as countries, subnational administrative units or selected facilities, where the HIV prevalence among adult pregnant women is ≥1% or among TB patients is ≥5% (18).
8 WHO does not intend to establish thresholds for low, moderate or high levels of prevalence of isoniazid resistance; NTPs should establish definitions for their own countries.

 Retour
Retour