Liens transversaux de livre pour 1266
Since 2007, the guideline development process within the WHO has been overseen by the WHO Guidelines Review Committee (GRC), which follows internationally recognized standards such as the GRADE approach [Grading of Recommendations Assessment, Development, and Evaluation], to support a structured and transparent methodology for policy-making. The policy recommendations presented in the guideline document were developed following the standards and updated procedures as described in the WHO Handbook for Guideline Development.
A WHO Guideline Steering Group was first established to determine specific areas requiring up-to-date evidence and to carry out arrangements to bring together experts to synthesize and independently review new evidence and develop recommendations. An external review group was also assembled to review the updated recommendations based on the inputs of the Guideline Development Group. The Guideline Development Group comprises researchers, epidemiologists, end-users (clinicians and national TB control programme officers), community representatives, and experts in evidence synthesis. In compliance with the procedures and practices established by the GRC, declarations of interest (DOI) were managed according to the WHO Conflict of Interest Policy, including a review of curriculum vitae and critical evaluation of DOI. Additionally, contingent on the assessment of competing interests, the full list of members of the Guideline Development Group and their biographies were published on the WHO website. This was followed by a public notice and comment period, during which WHO allowed members of the public to provide comments pertinent to any interests that may have gone unnoticed or not reported during earlier assessments.
During the virtual Guideline Development Group meeting, the members of the GDG reached decisions through a process of discussion and consensus. Where consensus through discussion could not be reached, the GDG voted on decisions. In these cases, decisions were made based on the vote of the majority.
Preparation for evidence assessment
The GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) was used to rate the certainty in the estimate of effect (quality of evidence) as high, moderate, low, or very low and to determine the strength of the recommendations (as strong or conditional). A scoping proposal was submitted and approved by the WHO Guideline Review Committee. Details about the preparatory work ahead of the update were released to the public through public comment, focusing on the following: (i) rationale for providing up-to-date guidance, including the scope of the updates; (ii) prioritization and formulation of key questions; as well as (iii) the list, affiliations, and constituencies of potential members of the Guideline Development Group, undergoing conflict of interest assessments, as per the WHO Office of Compliance, Risk Management and Ethics policies. In preparation for the Guideline Development Group meeting, four webinars (via Zoom) were held with members of the Group to finalize the scoping and PICO (Patients, Intervention, Comparator and Outcomes) questions, score outcomes of interest, and discuss preliminary data analysis results. The PICO questions, including sub-populations, treatment regimen composition, duration, and outcomes, were agreed upon by members of the Guideline Development Group. The questions were framed to capture the effect of novel treatment regimens for specific populations and the values, in terms of effectiveness and safety, of adding, prolonging and combining specific anti-tuberculosis agents.
The PICO questions looked at the following six distinct outcomes: (i) sustained treatment success; (ii) treatment failure and recurrence; (iii) death (due to any cause); (iv) loss to follow-up; (v) serious adverse event or adverse events of special interest; (vi) amplification (acquisition) of drug resistance. Members of the Guideline Development Group were invited to score the outcomes as “critical”, “important” or “not important for making recommendations on the use of specific regimens” under evaluation.
Certainty of evidence and strength of recommendations
In assessing the quality of evidence, several factors can increase or decrease the quality of evidence. The highest quality rating is usually assigned to evidence gathered from randomized control trials, while evidence from observational studies, including programmatic data, may usually assigned a low or very low-quality value. The higher the quality of evidence, the more likely a strong recommendation can be made (Table A2.1). The criteria used by the Guideline Development Group to determine the quality of available evidence are summarized in the Summary of Evidence tables. The certainty in the estimates of effect (quality of evidence) was assessed and either rated down or up based on: risk of bias; inconsistency or heterogeneity; indirectness; imprecision; and other considerations.
Table A2.1. Certainty of evidence and strength of recommendations

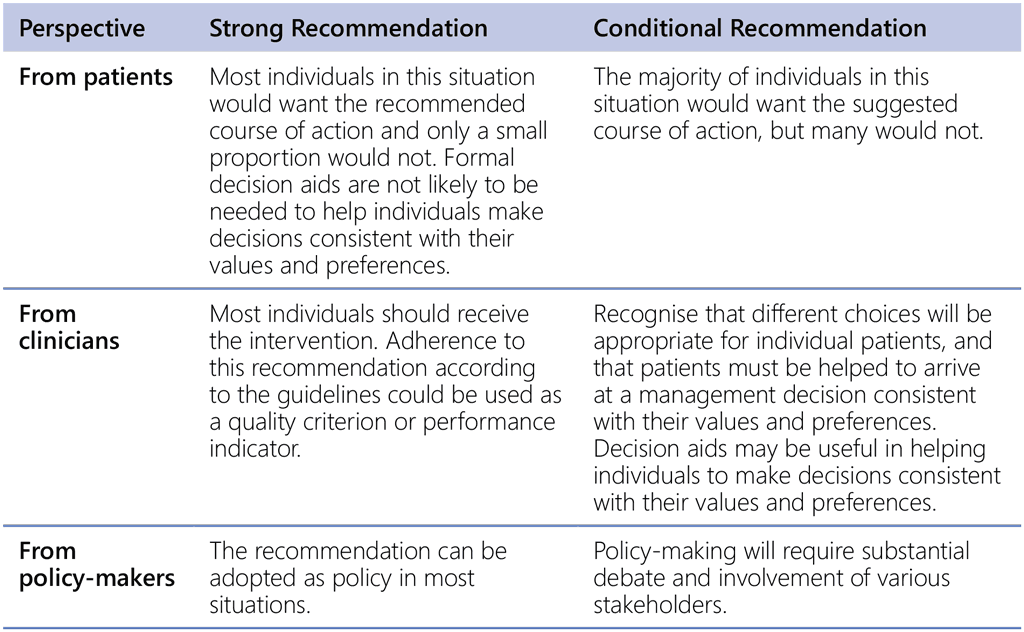
Through the GRADE system, the strength of a recommendation is classified as “strong” or “conditional”. The strength of a recommendation is determined by the balance between desirable and undesirable effects, values and preferences, resource use, equity considerations, acceptability and feasibility to implement the intervention. For strong recommendations, the GDG is confident that the desirable effects of adherence to the recommendation outweigh the undesirable effects. For conditional recommendations, the GDG considers that desirable effects probably outweigh the undesirable effects. The strength of a recommendation has different implications for the individuals affected by these guidelines (Table A2.2).
Table A2.2. Perspective taken and description of strength and conditionality of recommendations
Assessment of the quality of the evidence
The WHO Guideline Development process uses specific criteria to assess the characteristics of a body of evidence, such as within-study bias (methodological quality), consistency, precision, directness or applicability of the evidence, and others.
Publication, implementation, evaluation and expiry
These guidelines were prepared in accordance with the requirements of the Guideline Review Committee. The guidelines will be published on the WHO website for free download as part of comprehensive WHO consolidated guidelines on tuberculosis and will be communicated widely at international and regional conferences and meetings of programme managers in all regions. In parallel with the guidelines, WHO will also release an operational handbook with more practical details to support the programmatic implementation of the new or revised recommendations. National programmes will be supported by WHO and technical and funding partners to prepare a national plan for the programmatic management of drug-resistant TB. Implementers should create a conducive policy and programmatic environment, including national and local policies and standard operating procedures, to facilitate the implementation of the recommendations in these guidelines. This should include promoting universal health coverage and offering public financing for DR-TB management. Furthermore, dedicated resources should be allocated, including for staff development and service delivery in the community. Training of frontline healthcare staff and students on critical areas such as diagnosis, designing a regimen, patient support, monitoring response to treatment and management of adverse reactions is important. National programmes should ensure meaningful engagement with affected populations, their communities, the private sector, and other relevant health programmes and ministries in both planning and implementing the recommendations. The uptake of these WHO recommendations will be monitored in the annual data collection of WHO Global TB Data Monitoring.

 Retour
Retour