Liens transversaux de livre pour 1266
This section refers to an Hr-TB treatment regimen that has a duration of 6 months and uses oral agents.
WHO released its first evidence-based guidance for the treatment of Hr-TB using the GRADE approach in 2018 (1). The guidance is based on these two recommendations:

The recommendations made were conditional (4) and had very low certainty of evidence.
The basic regimen can be summarized as:

All medicines in this regimen are to be used daily for 6 months. When FDC formulations are used, isoniazid is included but it is not obligatory for the regimen. If levofloxacin cannot be used because there is FQ resistance or intolerance or other contraindications to the use of FQ, then 6(H)RZE may be prescribed daily for 6 months.
7.1 Eligibility
The Hr-TB regimen is recommended once isoniazid resistance has been confirmed and rifampicin resistance excluded. Rifampicin resistance needs to be excluded using rapid molecular tests (e.g. Xpert MTB/RIF) before levofloxacin is used, to avoid the inadvertent treatment of MDR/RR-TB with an inadequate regimen. Ideally, rapid DST for FQ and pyrazinamide is also performed.
It is not advisable to give a regimen for Hr-TB unless isoniazid resistance is confirmed or highly suspected (e.g. confirmed TB patient who is the close contact of a documented Hr-TB case). This will avert the unnecessary use of levofloxacin and prolonged pyrazinamide exposure in TB patients who may be cured with 2HRZE/4HR. Once the Hr-TB regimen has been started, if the results of initial DST reveal isoniazid susceptibility, the regimen may be modified so that the patient effectively completes a course of first-line TB treatment.
The recommendations apply to both adults and children, including PLHIV. Thus, HIV testing and treatment of PLHIV with ART is important, and the aim is to start ART within 8 weeks of TB treatment initiation (regardless of CD4 count), or within the first 2 weeks in patients with profound immunosuppression (e.g. CD4 counts <50 cells/mm³ ) (128). The regimen is also likely to be effective in patients with extrapulmonary Hr-TB; however, consultation with appropriate specialists is advised.
Hr-TB treatment is expected to be started if either of the following circumstances apply:
- Hr-TB is confirmed and rifampicin resistance is ruled out before TB treatment is started – in such cases, the 6(H)RZE-Lfx regimen is started immediately. If the diagnosis is strongly presumed (e.g. close contact of a confirmed Hr-TB source case) but results of DST are still pending, the regimen may be introduced. Should DST results taken at the start eventually show susceptibility to isoniazid, then levofloxacin is stopped and the patient continues treatment to complete a 2HRZE/4HR regimen.
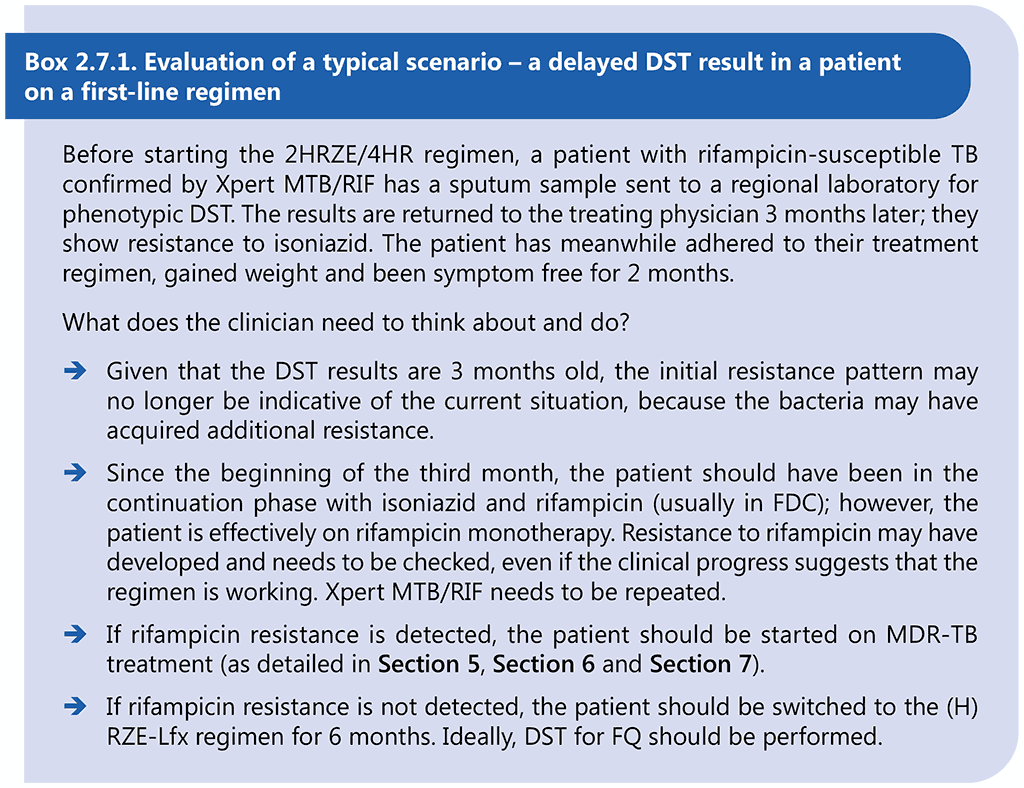
- Hr-TB is discovered after the start of treatment with the 2HRZE/4HR regimen (this includes patients who had undiagnosed isoniazid resistance at the start or who developed isoniazid resistance while on first-line treatment) – in such cases, rapid molecular testing for rifampicin resistance must be undertaken (or repeated). Once rifampicin resistance has been excluded, a full 6-month course of (H)RZE-Lfx is given. The duration is driven by the need to give levofloxacin for 6 months, which usually implies that the companion first-line medicines are taken for longer than 6 months. A report of resistance during treatment presents the clinician with a challenge, because the results may no longer reflect the drug susceptibility of the current bacterial population, given that an inadequate regimen – at times a functional monotherapy – may have favoured the acquisition of additional resistance in the interval. The unexpected discovery of resistance to one agent should prompt the clinician to repeat DST for other agents in the regimen. The example in Box 2.7.1 illustrates a typical situation that could arise.
7.2 Composition and duration of the regimen
The duration of Hr-TB treatment is driven by the need to complete 6 months of a FQ-containing regimen. This implies that, when Hr-TB is diagnosed after the start of the regimen for treatment of DS-TB, the companion medicines (HRZE) would end up being given for more than 6 months.
In patients with cavitary disease and with persistent positivity on sputum smear and culture, prolongation of (H)RZE-Lfx beyond 6 months could be considered on a case-by-case basis. Prolongation of treatment increases the risk of toxicity, particularly from pyrazinamide and ethambutol, which are usually only given for 2 months in the first-line TB regimen. The evidence reviewed for the WHO guidance on Hr-TB precluded a recommendation to limit the pyrazinamide duration to less than 4 months when a FQ is given.
Levofloxacin is the preferred FQ for Hr-TB regimens. The exposure to moxifloxacin decreases markedly when it is combined with rifampicin (129). This effect has not been reported in the case of levofloxacin; also, levofloxacin appears to cause less QT interval prolongation than moxifloxacin (55, 130, 131).
Levofloxacin is included in Hr-TB regimens except in the following instances: when rifampicin resistance cannot be tested for, when there is documented resistance or known intolerance to FQ, and when there is preexisting prolongation of the QT interval and pregnancy. If a FQ cannot be used, a patient with Hr-TB can still be treated with 6(H)RZE; streptomycin is not required in such cases.
For patient convenience and ease of administration, the HRZE FDC may be used to treat Hr-TB (given that no RZE FDC is currently available). The dosage of other TB medicines in the Hr-TB regimen is the same as in the standardized DS-TB 2HRZE/4HR regimen. The inclusion of isoniazid in the regimen has not been shown to lead to substantial benefit or harm to patients; however, isoniazid may increase the hepatotoxicity of pyrazinamide (132, 133). High-dose isoniazid (10–15 mg/kg per day) may still be effective when used in combination regimens in the presence of isolated inhA mutations linked to low MIC, even in “fast acetylators” (i.e. individuals who metabolize isoniazid rapidly) (134). In the presence of both inhA and katG mutations, addition of isoniazid (even at a high dose) is unlikely to add value to the regimen.
Patients with Hr-TB may have a higher risk of acquiring additional resistance and MDR-TB, which may manifest during the same treatment episode or in a subsequent relapse. The effect of additional resistance to ethambutol and pyrazinamide on the treatment of Hr-TB is unclear.
7.3 Considerations for implementation
The regimens recommended for treatment of Hr-TB is not divided into an intensive and a continuation phase – this simplifies the delivery and monitoring of treatment. Treatment is given daily, and intermittent treatment should be avoided. Relevant measures to support adherence, social support and the use of digital technologies should be considered to ensure favourable treatment outcomes (17).
The cost of medicines to compose a full 6(H)RZE regimen with levofloxacin is slightly higher than the cost of a 2HRZE/4HR regimen used for DS-TB (135). Nonetheless, the 6(H)RZE regimen is an affordable and feasible intervention, even in low-income settings. Use of FDCs simplifies treatment and lowers costs, and the use of dispersible formulations of HRZ, ethambutol and levofloxacin is preferred in children. As with the treatment of other forms of TB, the expenses associated with the proper delivery of care (e.g. DST, adherence support and clinical monitoring) far exceed the cost of medicines.
A new diagnostic platform has been approved for the detection of Hr-TB – the new Xpert MTB/XDR cartridge, which can detect isoniazid resistance in less than 90 minutes, matching the rapidity and convenience of Xpert MTB/RIF for rifampicin resistance. First-line LPA can also detect isoniazid resistance, and the infrastructure required is typically available in a provincial or central level facility. Typical processing time for an LPA specimen is about 2–3 days, owing to batching. DST based on liquid culture (or MGIT) could also detect Hr-TB at the level of a reference laboratory, but this means a processing delay of at least 10 days. Testing on solid media is also an option, but it takes several months to obtain results; hence, this approach is of limited use for baseline testing and monitoring of treatment response.
Current epidemiological data indicate that more than three quarters of the global burden of Hr-TB occurs among previously untreated (“new”) TB cases. Previous TB treatment is thus not a strong indicator of risk of Hr-TB – the correlation with previous TB treatment is weaker than it is with MDR-TB. Reserving isoniazid DST to such patients is therefore unlikely to yield many Hr-TB cases. There are various concerns about empirical Hr-TB treatment of previously treated TB cases, without prior DST. First, such treatment will lead to unnecessary overtreatment with FQ and prolongation of pyrazinamide use in many patients. Most recurrent cases will not have Hr-TB and can be cured with a 2HRZE/4HR regimen. Second, unless rifampicin resistance is excluded at the baseline, patients with MDR/RR-TB would be exposed to an inadequate regimen, with the risk of acquiring additional resistance, including FQ. Third, this policy would deflect the focus of the programme from testing new TB patients, who usually harbour the main burden of Hr-TB. Finally, this approach would risk creating once again a “re-treatment regimen”, similar to the situation that prevailed in many settings until recently with the indiscriminate use of the streptomycin-containing 8-month “Category 2” regimen in all previously treated TB patients.
In a situation where access to DST is good, a logical diagnostic algorithm would start with Xpert MTB/RIF as the initial test for all patients evaluated for TB. Cases in whom TB is confirmed and rifampicin resistance is not detected would be further tested with Xpert MTB/XDR or LPA. Liquid culture may replace LPA, but the additional delay in obtaining results is a disadvantage.
7.4 Treatment monitoring
The clinical monitoring of patients on Hr-TB treatment follows similar principles to those that apply to other first-line TB regimens. Bacteriological monitoring of sputum generally follows the same schedule as DS-TB, with direct microscopy at months 2, 5 and 6. It is desirable, however, to perform a culture together with smear microscopy (or at least in the last month of treatment) to check for any emergent resistance, especially to rifampicin. Non-response to treatment should be investigated with DST.
Liver and kidney function and other blood tests may be necessary, based on clinical manifestations and medications in use. ECG for patients on 6(H)RZE-Lfx is not usually required unless there are other risks for QT interval prolongation. The first-line TB agents may cause adverse drug reactions, which are mostly mild, not serious and self-limiting or manageable with basic measures. TB practitioners are likely to be more familiar with the use of these medicines than with levofloxacin, which has a fairly good safety profile in both adults and children when used at the dose recommended in the Annex 4, even when taken for longer than 6 months. Dosage adjustment, in consultation with a specialist, is recommended if creatinine clearance is below 30 mL/min (15). Adverse drug reactions should be reported to the spontaneous pharmacovigilance systems required by national regulations, as for other drug-related harms. In patients on regimens for Hr-TB, aDSM is not mandatory.
As with all other notifiable TB cases, patients with Hr-TB should be registered in the TB register, regardless of whether treatment has started, or whether a regimen containing second-line TB medicines is being given (113). The case may be retained in the TB register for the purposes of monitoring the treatment response and the interim or final outcomes. Cases without Hr-TB may be enumerated with the main DS-TB cases for the purposes of treatment outcome reporting. Hr-TB cases given FQ or other second-line agents in addition to 6(H)RZE may also be registered in the second-line TB register if the programme wishes to monitor how many patients are being given regimens containing second-line medicines (15). If this is done, it is important that cases without RR-TB are not enumerated with the MDR/RR-TB cohort for treatment outcome monitoring purposes.
It will be helpful to monitor efforts to improve testing coverage, detection, enrolment and outcomes for Hr-TB separately from other TB or MDR/RR-TB cases. The indicators for MDR/RR-TB may be adapted for this purpose; outcome definitions are the same as for non-MDR/RR-TB (113). Reporting can be at the same frequency as that recommended for standard monitoring of other TB cohorts.
Combining data for patients with different resistance patterns into a single cohort may complicate comparison of performance between centres and determination of trends over time, given that these patients may have different risks for treatment failure. However, treatment of TB patients who do not have rifampicin resistance with regimens discussed in this section should lead to a successful outcome in most patients, and maximizing the likelihood of success should be the end objective of TB programmes. The use of electronic case-based databases facilitates the grouping of patients by comparable resistance patterns or treatment episodes to undertake more advanced analyses, allowing adjustment for at least some covariates. Programmes are encouraged to follow good practices when collecting these data, and to participate in collaborative initiatives to share individual patient records for pooled reviews of global patient series (136–139). Such data could be useful to guide future policy on the optimization of regimens for the treatment of DR-TB.

 Retour
Retour