Liens transversaux de livre pour 1266
The desired outcomes from diagnostic tests that are implemented are that the test should:
- provide accurate results;
- provide timely results to impact clinical decision-making;
- be justified based on need; and
- be quality assured, reliable and reproducible.
The decision on where to place a specific test is an important one because it can lead to success or failure in achieving these desired outcomes. Also, a diagnostic test should not be seen in isolation from the broader ecosystem of tests (TB and non-TB) used to deliver results for clinical management. To support these decisions, WHO and the GLI have published a manual for selection of mWRDs, for programme reference (15).
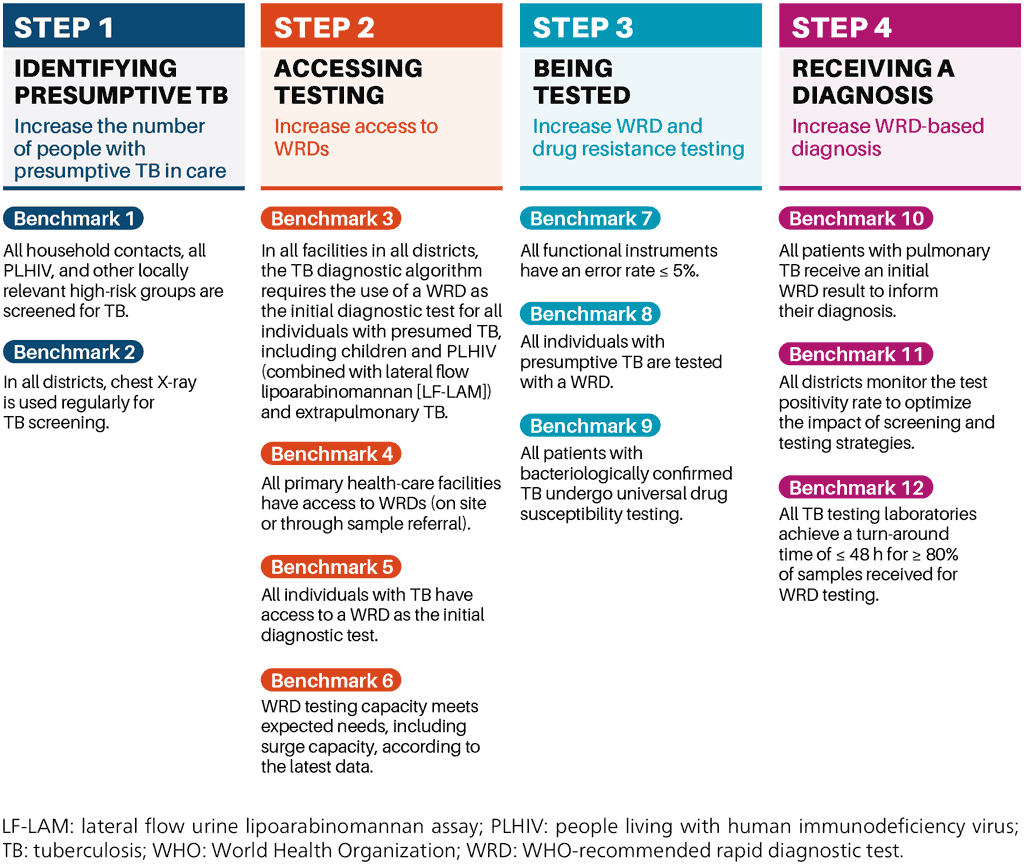
The WHO standard: universal access to rapid tuberculosis diagnostics (3) can serve as a guide for implementing and monitoring improvements to TB testing and diagnostic networks, to achieve universal access. The standard comprises 12 benchmarks across four steps (Fig. 4.1), requiring a holistic approach to diagnostic delivery that comprises laboratories, health facilities and individual patients.
In many resource-limited or high-burden settings, TB laboratory networks have a pyramidal structure, as shown in Fig. 4.2. This structure has the largest number of laboratories at the peripheral level (Level I); a moderate number of intermediate laboratories (Level II), usually located in mid-sized population centres and health facilities; and a single central laboratory (Level III) – or more than one in large countries – at the provincial, state or national level. Each level or tier has specific requirements for infrastructure and biosafety, defined by a risk assessment, as outlined in the WHO biosafety manual for TB laboratories (60).
Fig. 4.1. The 12 benchmarks constituting the WHO standard are divided into four steps along the diagnostic cascade

 Retour
Retour