Liens transversaux de livre pour 1266
Algorithm 2a and 2b are used for further evaluation of people with MDR/RR-TB. In its most recent recommendations (7), WHO strongly encourages DST in people with MDR/RR-TB, although that should not delay treatment initiation. DST should be performed as soon as possible and even if results are not available at the start of the selected regimen (preferably BPaLM or BDLLfxC), the results should be used to adjust it. Two of the key medicines in these regimens are BDQ and FQ. Two WHO-recommended molecular tests to detect mutations associated with BDQ resistance belong to the class of targeted NGS (Deeplex® Myc-TB from GenoScreen and AmPORE-TB from Oxford Nanopore Diagnostics). Algorithm 2a relies on testing using the targeted NGS test to detect mutations associated with resistance to BDQ, FQ, LZD and other medicines used in the recommended regimens. Because of the limited availability of targeted NGS tests at this time, a second algorithm (Algorithm 2b) is included that relies on the detection of mutations associated with FQ resistance using mWRDs (LC-aNAAT and SL-LPA) and phenotypic DST for BDQ and other drugs. WHO stresses the need to scale up laboratory phenotypic DST capacity for medicines for which there are accurate and reproducible phenotypic methods, including BDQ, LZD, Pa, CS, CFZ and DLM.
6.4.1 Decision pathway for Algorithm 2 – DST for second-line drugs for people with MDR/RR-TB
General considerations
Seven BDQ-containing 6- or 9-month regimens are recommended for the treatment of MDR/RR-TB (7, 95):
- Two all-oral 6-month regimens: BPaLM (for individuals aged 14 years and older) composed of BDQ, Pa and LZD, with MFX, which is dropped in case FQ resistance is detected; and BDLLfxC composed of BDQ, DLM, LZD, LFX and CFZ, for which LFX is dropped if FQ resistance is detected, CFZ is dropped if FQ susceptibility is confirmed, and both are retained if FQ status cannot be determined. This latter regimen is suitable for use in children and pregnant and lactating women because Pa is not currently recommended for use in these groups.
- Five all-oral regimens of 9 months comprising different combinations of BDQ, LFX or MFX, LZD, CFZ, DLM and PZA (BLfxEtoEHZC, BLfxLEHZC, BLMZ, BLLfxCZ and BDLLfxZ).
Individualized all-oral longer regimens, designed using the WHO priority grouping of medicines, may still be used for people with MDR/RR-TB and FQ resistance who do not meet the eligibility criteria for the 6-month and 9-month regimens.
Fig. 6.3 provides an overview of the treatment regimens recommended by WHO. Several factors matter when a regimen is selected, as detailed in WHO guidelines (7, 95). Common to all MDR/RR-TB regimens is that BDQ is the central drug. An individual with a confirmed resistance to BDQ should be switched to an individualized regimen and drugs should be selected based on targeted NGS results and adjusted based on phenotypic DST results.
Fig. 6.3. Treatment option overview
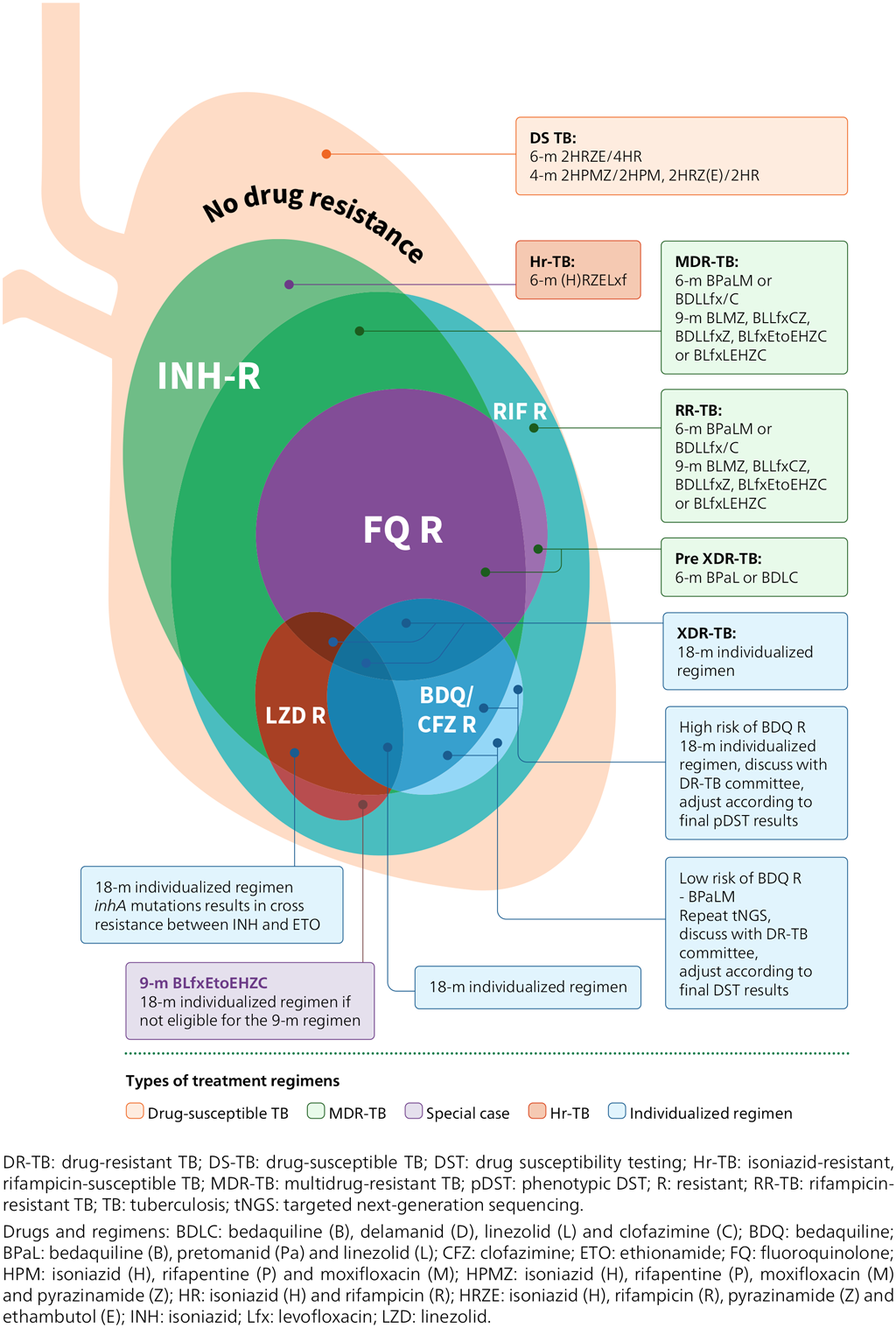
- WHO guidelines stress the importance of DST, especially for medicines for which molecular tests are available; however, it is important that the initiation of treatment is not delayed while waiting for DST results. The selected regimen should be adjusted if needed, based on DST results that may become available after initiation of treatment.
- WHO recommends molecular tests for the detection of mutations associated with resistance to FQs (LC-aNAAT, SL-LPA and targeted NGS tests) and mutations associated with BDQ resistance (targeted NGS tests). Targeted NGS tests can also detect mutations associated with resistance to some of the other drugs included in MDR-TB regimens (e.g. LZD, CFZ and PZA).
- WHO recommends a molecular test for PZA resistance detection belonging to the class “HC-rNAAT”. Its use is limited to culture isolates. Alternatively, pncA sequencing should be performed when available.
- In settings where targeted NGS is not available, phenotypic DST should be used.
- In a quality-assured laboratory, with careful attention to inoculum preparation, a susceptible phenotypic DST result using MGIT for PZA can be used to guide the inclusion of PZA in a DR-TB treatment regimen (Web Annex C).
- Reliable phenotypic DST methods are available for RIF, INH, FQs, BDQ, CFZ, Pa, CS, LZD, AMK and DLM. Testing algorithms that rely on culture and phenotypic DST are described in the relevant WHO policy framework (96) and technical manual (Web Annex C). Member States should ensure that there is capacity for DST for drugs used for treatment and for which reliable testing is available.
- No reliable phenotypic DST methods are available for EMB, ETO/prothionamide, or imipenem-cilastatin/MPM; hence, results should not be used for clinical decision-making.
- If phenotypic DST to second-line drugs is not available in-country, specimens or isolates may be shipped to an external laboratory for testing (e.g. a WHO supranational reference laboratory [SRL]). Material transfer agreements and import or export permits may be needed.
- Currently, the availability of phenotypic DST for BDQ and LZD is limited in many settings, and resistance levels are likely to be low. There is, however, increasing evidence that BDQ resistance occurs even in unexposed people at a level of 1.4–3.4% (97). BDQ is a core drug for DR-TB treatment and is included in the revised definition of XDR-TB. Thus, it is essential to build testing capacity to test this and other drugs used in treatment (e.g. LZD, Pa, CS, CFZ and DLM). If resistance is suspected during treatment and DST is not available, the strains should be referred to a TB SRL for further testing.
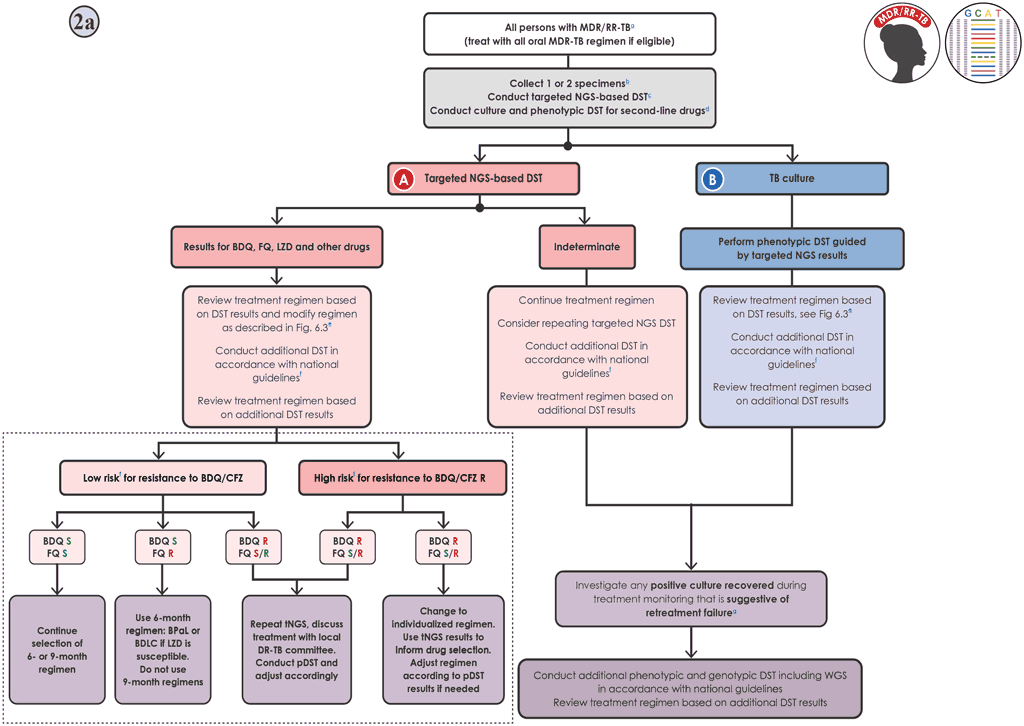
Fig. 6.4. Algorithm 2a: DST for MDR/RR-TB using targeted NGS
DR-TB: drug-resistant TB; DST: drug susceptibility testing; MDR/RR-TB: multidrug- or rifampicin-resistant TB; MDRTB: multidrug-resistant TB; NGS: next-generation sequencing; pDST: phenotypic DST; RR-TB: rifampicin-resistant TB; SRL: supranational reference laboratory; TB: tuberculosis; tNGS: targeted next-generation sequencing; WGS: whole-genome sequencing; WHO: World Health Organization.
Drugs and regimens: BDQ: bedaquiline; BPaL/M: bedaquiline (B), pretomanid (Pa) and linezolid (L), and moxifloxacin (M); CFZ: clofazimine; CS: cycloserine; DLM: delamanid; EMB: ethambutol; FQ: fluoroquinolone; INH: isoniazid; LZD: linezolid; Pa: pretonamid; PZA: pyrazinamide; RIF: rifampicin; STR: streptomycin.
a Patients should be promptly initiated on an MDR/RR-TB regimen in accordance with national guidelines and WHO recommendations. A shorter all-oral bedaquiline-containing treatment regimen (BPaL/M or BDLLfxC) is the preferred option for eligible people with MDR/RR-TB.
b If molecular and phenotypic testing are performed in the same laboratory, one specimen may be sufficient. If testing is performed in two laboratories, two specimens should be collected, and the molecular and phenotypic testing conducted in parallel.
c WHO recommends performing DST using rapid molecular tests among all people diagnosed with TB, although this testing should not delay the start of treatment. Currently, targeted NGS tests can provide results for BDQ, FQ, LZD, INH, PZA, EMB, CFZ, AMK, STR and RIF.
d Phenotypic DST should be conducted for each of the drugs included in the treatment regimen for which there are accurate and reproducible methods. Reliable phenotypic DST methods are available for RIF, INH, FQs, PZA, BDQ, CFZ, Pa, CS, LZD, AMK and DLM. The initiation of treatment should not be delayed while awaiting the results of the phenotypic DST.
e More details on individualized regimens can be found in the WHO consolidated guidelines on tuberculosis: module 4: treatment (7).
f Low risk for BDQ/CFZ resistance is when there is no prior BDQ/CFZ exposure, and the prevalence of resistance to BDQ/CFZ is less than 5% in the population (people with RR-TB on national or subnational level where reliable estimates are available), and there is no history of contact with a person known to have BDQ/CFZ-resistant TB. If any of these conditions are not met the risk is considered high.
g If resistance to an individual drug (e.g. BDQ) is suspected and DST for this drug is not available in the country, laboratories will need to have mechanisms to store the isolate and ship it to a WHO SRL for DST.
Decision pathway for Algorithm 2a – Testing for BDQ and FQ resistance (Fig. 6.4)
- The person should be promptly initiated on an MDR-TB regimen in accordance with national guidelines. The most recent WHO recommendations include two different all-oral 6-month BDQ-containing regimens (7, 95). In addition, five all-oral 9-month regimens are recommended by WHO and can be used if neither of the 6-month regimens are suitable (7, 95).
- If molecular and phenotypic testing are performed in the same laboratory, collecting one specimen may be sufficient. If testing is performed in two laboratories, two specimens should be collected and the molecular and phenotypic testing conducted in parallel. Sputum specimens or isolates should be transported to the appropriate testing laboratory, if necessary.
- Targeted NGS testing should be conducted to detect mutations associated with resistance to BDQ and other medicines, and culture and DST should be undertaken for drugs not included in the targeted NGS solution used, in parallel 2aA.
- If the targeted NGS test result is indeterminate, the test can be repeated with a fresh sample in cases where the bacterial load is expected to give a definitive result (smear positive or high or medium grade on mWRD); treatment decisions should be based on clinical assessment, the epidemiological situation and the results of phenotypic DST. The results of the targeted NGS test should be used to modify treatment if appropriate, and to select the drugs included in the regimen requiring phenotypic DST when the culture is positive 2aB. Pa and DLM are not covered by any targeted NGS solutions and require phenotypic DST.
- When selecting or designing the treatment regimen, the results of the DST for all drugs should be taken into consideration (Fig. 6.3). Further details on regimens are given in Module 4 of the WHO consolidated guidelines (7).
- BDQ is a core drug of all 6-month and 9-month MDR/RR-TB regimens, and the interpretation of the targeted NGS results has important implications for treatment choices. It is necessary to evaluate the pretest probability of BDQ resistance before making clinical decisions, because of the suboptimal performance of the targeted NGS test for detecting BDQ resistance (sensitivity: 67.9%, 95% CI: 42.6–93.2%; specificity: 97.0%, 95% CI: 94.3–99.7%). A low risk for BDQ/CFZ resistance is when there is no prior BDQ/CFZ exposure, AND the prevalence of resistance to BDQ/CFZ is less than 5% in the population, AND there is no history of contact with a person known to have BDQ/CFZ-resistant TB. If any of these conditions are NOT met the risk is considered high.
- If the risk of BDQ resistance is low and the targeted NGS test does not detect mutations associated with resistance to BDQ, the negative predictive value will be high, and the result is likely to be true. In cases of FQ resistance, one of two 6-month regimens should be used but the FQ should be dropped (i.e. BPaL or BDLC [BDQ, DLM, LZD and CFZ] should be used, assuming that tests show susceptibility to LZD). The 9-month regimens recommended by WHO should not be used. In cases of susceptibility to FQ, the selected 6-month or 9-month regimen should be continued, or one of the other 6-month or 9-month regimens should be adapted based on the full targeted NGS profile. The decision to perform phenotypic DST for BDQ for this group will be context dependent, taking into account the prevalence of resistance, DST capacity and the expected number missed by targeted NGS. Ideally, where resources are available, phenotypic DST should be done for all samples. The regimen should be modified as appropriate if resistance to other medicines in the regimen is detected.
- In situations with low pretest probability of BDQ resistance and where the targeted NGS test detects one or more mutations associated with resistance to BDQ, the targeted NGS should be repeated, the treatment decision discussed with the local DR-TB committee, phenotypic DST performed and adjustments made accordingly. Note: Rv0678 mutations detected and associated with BDQ resistance usually result in cross-resistant to CFZ because they share a common mechanism; furthermore, the accuracy of targeted NGS for CFZ is similar to that for BDQ.
- In a situation with a high pretest probability of BDQ/CFZ resistance, where the targeted NGS test does not detect mutations associated with resistance to BDQ, the targeted NGS should be repeated and treatment discussed with the local DR-TB committee based on risk factors, to make a decision on treatment while awaiting phenotypic DST results. The number of true susceptible cases will be much higher than the number of false susceptible cases.
- If the risk of BDQ resistance is high and the targeted NGS test detects one or more mutations associated with resistance to BDQ, the person should be given an individualized regimen that is based on targeted NGS and adjusted according to phenotypic DST results. As noted above, Rv0678 mutations detected and associated with BDQ resistance usually result in cross-resistant to CFZ because they share a common mechanism; furthermore, the accuracy of targeted NGS for CFZ is similar to that for BDQ.
- People should be closely monitored, and additional DST performed on any culture isolated at month 2 or later during treatment.
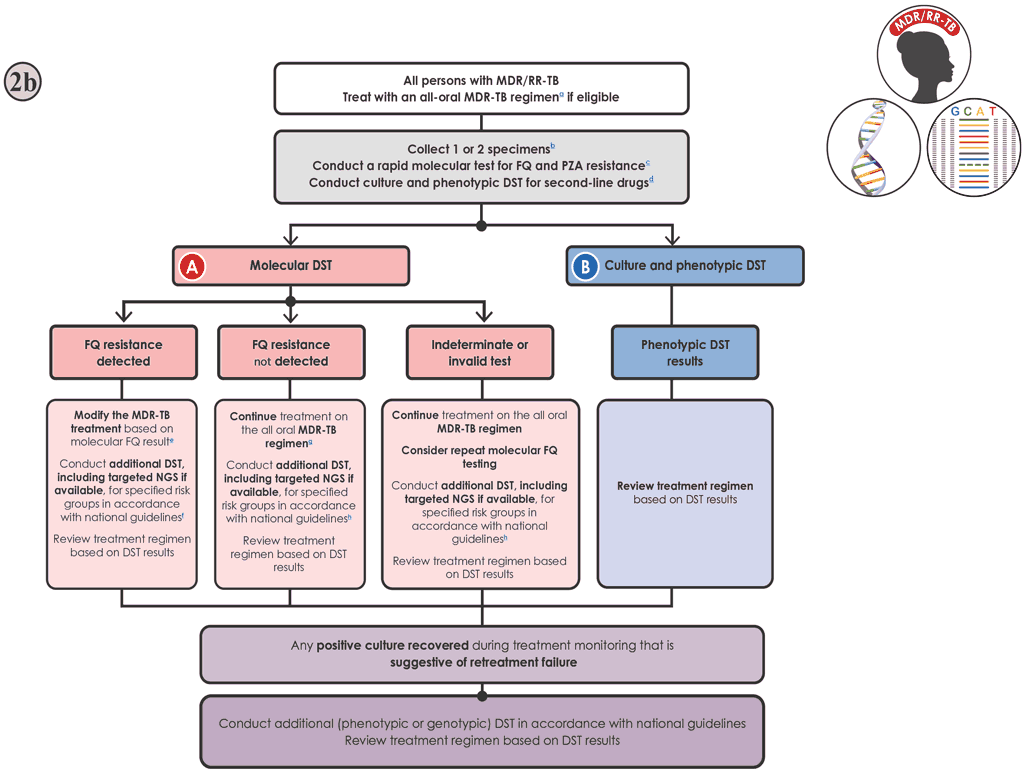
Fig. 6.5. Algorithm 2b: DST for people with MDR/RR-TB (limited or no targeted NGS capacity)
DR-TB: drug-resistant TB; DST: drug susceptibility testing; MDR/RR-TB: multidrug- or rifampicin-resistant TB; MDR-TB: multidrug-resistant TB; mWRD: molecular WHO-recommended rapid diagnostic test; NGS: nextgeneration sequencing; SL-LPA: line probe assay for second-line drugs; SRL: supranational reference laboratory; TB: tuberculosis; WHO: World Health Organization.
Drugs and regimens:
AMK: amikacin; BDLC: bedaquiline (B), delamanid (D), linezolid (L) and clofazimine (C); BDQ: bedaquiline; BDLLfx/C: bedaquiline (B), delamanid (D), linezolid (L), levofloxacin (Lfx) and clofazimine (C); BPaL: bedaquiline (B), pretomanid (Pa) and linezolid (L); BPaLM: bedaquiline (B), pretomanid (Pa), linezolid (L) and moxifloxacin (M); CFZ: clofazimine; CS: cycloserine; DLM: delamanid; EMB: ethambutol; FQ: fluoroquinolone; INH: isoniazid; LZD: linezolid; Pa: pretonamid; PZA: pyrazinamide; RIF: rifampicin; STR: streptomycin.
a People suspected of having TB should be promptly initiated on an MDR-TB regimen in accordance with national guidelines and WHO recommendations. An all-oral BDQ-containing treatment regimen (BPaLM or BDLLfx/C) is the preferred option for eligible people with MDR/RR-TB.
b If molecular and phenotypic testing are performed in the same laboratory, one specimen may be sufficient. If testing is performed in two laboratories, two specimens should be collected, and the molecular and phenotypic testing conducted in parallel.
c WHO recommends getting the rapid DST results before the start of treatment, although this testing should not delay the start of treatment. The mWRDs for detecting FQ resistance include Xpert MTB/XDR and SL-LPAs and the Genoscholar PZA-TB II test for detecting PZA resistance. Also, targeted NGS tests can provide results for BDQ, FQ, LZD, INH, PZA, EMB, CFZ, AMK, STR and RIF.
d Phenotypic DST should be conducted for each of the drugs included in the treatment regimen for which there are accurate and reproducible methods. Reliable phenotypic DST methods are available for RIF, INH, FQs, PZA, BDQ, CFZ, Pa, CS, LZD, AMK and DLM. The initiation of treatment should not be delayed while awaiting the results of the phenotypic DST.
e For more information regarding modifications of the treatment regimen, see the WHO consolidated guidelines on tuberculosis: module 4: treatment (7). BPaL or BDLC may be used for people with FQ-resistant MDR-TB (7). See Fig. 6.3
f For FQ-resistant MDR/RR-TB, a specimen should be collected and submitted for phenotypic DST to the WHO Group A (BDQ, Pa and LZD) and B drugs, if not already being done as described in Note 4 below. If targeted NGS tests are available, a sample should be submitted for testing for resistance to additional medicines for specified risk groups in accordance with national guidelines.
g In settings with a high underlying prevalence of resistance to FQs or for people considered at high risk of FQ resistance, a specimen should be referred for culture and phenotypic DST for FQs.
h If resistance to an individual drug (e.g. BDQ) is suspected and DST for these drugs is not available in the country, laboratories should establish mechanisms to store the isolate and ship it to a WHO SRL for DST.
Decision pathway for Algorithm 2b – Testing for FQ resistance (Fig. 6.5)
- The person should be promptly initiated on an MDR-TB regimen in accordance with national guidelines (see “General considerations” above).
- If molecular and phenotypic testing are performed in the same laboratory, collecting one specimen may be sufficient. If testing is performed in two laboratories, two specimens should be collected and the molecular and phenotypic testing should be conducted in parallel. Sputum specimens or isolates should be transported to the appropriate testing laboratory, if necessary.
- LC-aNAAT or SL-LPA should be conducted to detect mutations associated with FQ resistance. Targeted NGS tests can also detect mutations associated with resistance to FQ. If a targeted NGS test is available, Algorithm 2a should be followed for interpretation of results and follow-up actions.
- If the LC-aNAAT or SL-LPA detects one or more mutations associated with resistance to FQs and:
- the individual is on a BPaL/M regimen, MFX should be discontinued and BPaL treatment continued while awaiting the results of the phenotypic DST;
- the individual is on a BDLLfx/C, the LFX should be discontinued and BDLC continued (if the individual is FQ susceptible, C should be dropped from the regimen);
- the individual is on a 9-month all-oral regimen, the person should be moved to an individualized longer regimen, designed using the WHO priority grouping of medicines (7):
- the first-in-class LC-aNAAT (Xpert MTB-XDR) provides results for INH, FQs, ETO and AMK, and can be used to inform individualized regimen selection;
- a specimen should be collected and submitted for phenotypic DST to the WHO Group A, B and C drugs (e.g. for BDQ, Pa and LZD), if phenotypic DST is not already being done as described in Step 6; and
- DST for MFX should be performed at the clinical breakpoint to determine the potential use of high-dose (800 mg) MFX for treatment (7) (Web Annex C).
- If the LC-aNAAT or SL-LPA is negative for mutations associated with resistance to FQs (i.e. susceptible) and:
- the individual is on a BPaL/M regimen, treatment should continue without modifications while awaiting the results of the phenotypic DST;
- the individual is on a BDLLfx/C regimen, then C should be discontinued but BDLLfx continued while awaiting the results of the phenotypic DST;
- the individual is on one of the five 9-month regimens, treatment should continue without modification while awaiting the results of the phenotypic DST (Step 6); and
- in settings with a high underlying prevalence of resistance to FQs or for people considered at high risk of resistance, a specimen should be referred for culture and phenotypic DST for FQs, because the sensitivity of the LC-aNAAT and SL-LPA to detect mutations associated with FQ resistance is about 93% and 86%, respectively; the phenotypic DST should include testing for resistance to the FQs used in the country, and it should also include testing at the clinical breakpoint to inform individualized drug selection; the regimen should be modified as necessary, based on the phenotypic DST results.
- Culture and phenotypic DST should be performed for each of the drugs included in the treatment regimen for which accurate and reproducible methods are available. For the preferred regimens, phenotypic DST methods that are reliable when performed in a qualityassured laboratory are available for BDQ, LZD, Pa, CS, FQs, CFZ, PZA and INH (Web Annex C). A WHO-recommended molecular test for PZA resistance detection is available (HC-rNAAT) but its use is currently limited to culture isolates.
- If the isolate is susceptible to all drugs, the person should be continued on the preferred MDR-TB regimen.
- If resistance to a drug is detected, Fig. 6.3 should be used to guide treatment modification. Given that results for phenotypic DST are slow, the person’s response should be reassessed when these results become available. The decision to change from a shorter to the longer MDR-TB regimen should consider the phenotypic DST result and clinical response. Monthly monitoring is important, and the person should be closely followed up.
- For all people with TB, it is important to ensure that treatment monitoring includes the collection of samples for culturing, as described in the WHO consolidated guidelines (7). Any positive culture suggestive of treatment failure should undergo phenotypic DST and WGS where available, with interpretation based on the WHO catalogue of mutations (23). The regimen should be modified as necessary, based on the results.
- WHO recommends that all people with TB being treated with an MDR-TB regimen be monitored for treatment response using sputum culture and sputum smear microscopy. It is desirable for sputum culture to be repeated at monthly intervals.
- Although the risk of treatment failure increases with each additional month without bacteriological conversion, no discrete cut-off point has been defined that could serve as a reliable marker of a failing regimen. The choice of cut-off point will depend on the clinician’s desire to minimize the risk of failure; in particular, to limit the risk of prolonging a failing regimen.

 Retour
Retour