Book traversal links for 1266
2.1.1 Background
Early detection and treatment for TB among people living with HIV is crucial for reducing morbidity and mortality. TB screening tools are designed to distinguish people with a higher probability of having TB disease, from those with a lower probability. Screening tests need to be followed by a diagnostic test, offered as part of a comprehensive clinical evaluation, to confirm or rule out TB disease (12). WHO recommends that people living with HIV are systematically screened for TB disease at each visit to a health facility. Initially, the four symptom screen was recommended by WHO for TB screening among people living with HIV, as part of the Guidelines for intensified tuberculosis casefinding and isoniazid preventive therapy for people living with HIV in resource-constrained settings, published in 2011 (33). WHO recommendations on TB screening among people living with HIV using CRP, CXR, with the possibility to read the CXR using CAD, and mWRDs were first published in the 2021 WHO consolidated guidelines on tuberculosis. Module 2: screening – systematic screening for tuberculosis disease (12). Therefore, there are now four recommended approaches to TB screening among people living with HIV, namely the W4SS, CRP, CXR (with the possibility of CAD for reading) and mWRDs.
2.1.2 Summary of evidence and rationale
A systematic literature review and individual participant data (IPD) meta-analysis was conducted in 2020 to review the accuracy of tools for TB screening among adults and adolescents with HIV, including the W4SS, CRP, CXR and mWRDs. Data were analyzed for all study participants, as well as for five different subpopulations (inpatients, outpatients on ART, outpatients not on ART, people with CD4 count ≤ 200 cells/µl, and pregnant women living with HIV) where disaggregated data was available. Key findings are summarized below; further details are published in the TB screening guidelines (12).
Recommendation 1: Systematic screening for TB among people living with HIV at every visit
This recommendation, which applies to people of all ages, was first published in 2011 in WHO’s Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings (33), and it remains in place. The GDG for the development of the 2021 updated WHO guidelines on TB screening placed high value on ensuring that TB is diagnosed early in this risk group, who have a high likelihood of having undetected TB and a high risk of poor health outcomes in the absence of early diagnosis and treatment (12).
Recommendation 2: WHO-recommended four symptom screen
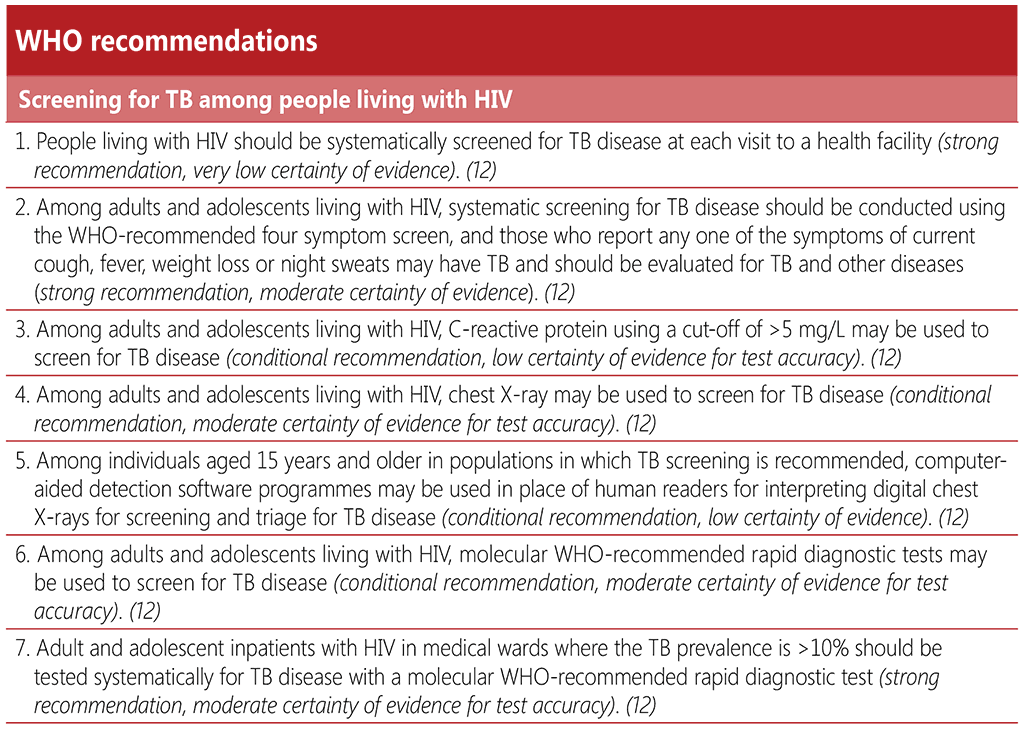
The 2020 IPD meta-analysis included 23 studies of 16 269 participants living with HIV, all of which reviewed the accuracy of the W4SS. The studies primarily focused on pulmonary TB disease. The unweighted average TB prevalence among participants within these studies was 9.2%, ranging from 1% to 26%; and 52% of people living with HIV screened positive on the W4SS. The sensitivity of the W4SS among all people living with HIV was 83% (95% CI: 74–89) and specificity was 38% (95% CI: 25–53). Estimates of the accuracy of the W4SS in different subgroups of people living with HIV are shown in Table 2.1. When used alone, the W4SS was found to have its lowest sensitivity among outpatients on ART and among pregnant women, and it had markedly low specificity among medical inpatients.
While there may be real-life limitations to the W4SS in terms of consistency that might not be reflected in studies, it remains the simplest non-invasive tool to implement in any setting, requiring no infrastructure. However, the high proportion of W4SS positivity (94%) and very low specificity in medical inpatients living with HIV in settings where TB prevalence among study participants was > 10% gives it limited utility as a screening tool to rule in TB prior to diagnostic confirmation by mWRD in this very ill population.
The IPD meta-analysis found no alternative screening tools or strategies that were significantly higher in both sensitivity and specificity than the W4SS or that met the WHO target product profile for a screening test on both parameters. In all cases, when sensitivity was higher and met the minimal requirements of the target product profile, specificity was compromised, and vice versa. Depending on a programme’s decision to prioritize higher sensitivity or higher specificity, other tools or combinations of tools may be used to complement the W4SS.
Table 2.1. Diagnostic accuracy of the WHO-recommended four symptom screen among different subpopulations of people living with HIV compared with culture as a reference standard
Recommendation 3: C-reactive protein
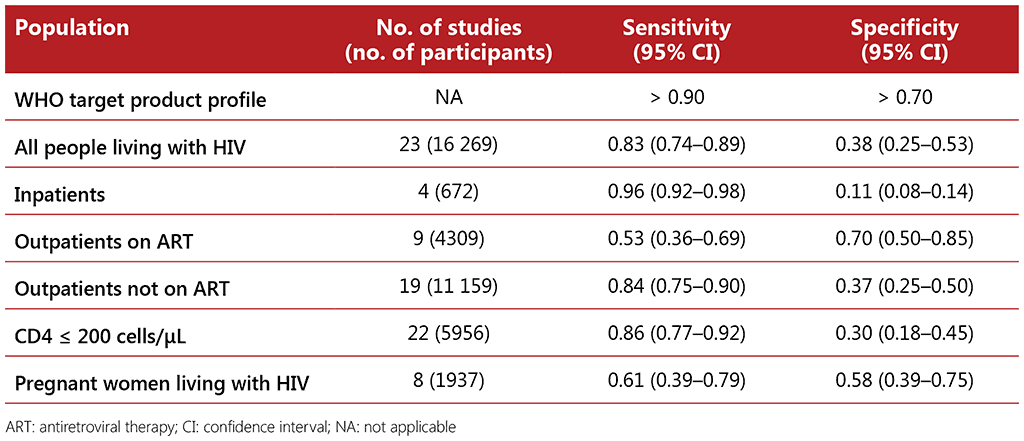
CRP is an indicator of general inflammation that can be measured using point-of-care tests performed on capillary blood collected via finger prick. The evidence reviewed for the performance of CRP included six studies from Kenya, South Africa and Uganda with a total of 3971 participants. The average unweighted prevalence of TB among participants in the studies was 14%, ranging from 1% to 26%.
Data on the accuracy of CRP using a cut-off value of > 5 mg/L and of > 10 mg/L as indicators of TB disease were reviewed and both cut-offs were considered to have similar or superior accuracy when compared with the W4SS. The cut-off of > 5 mg/L was recommended because it is the lowest threshold indicating abnormality in many clinical settings, and it has higher sensitivity than the cut-off of > 10 mg/L. The choice of cut-off will depend on the type of CRP technology available in a given setting, the prevalence of TB and of other conditions that may increase CRP and the preference for increased sensitivity or increased specificity.
The IPD meta-analysis on CRP using a cut-off of > 5 mg/L reported similar sensitivity to and higher or similar specificity to the W4SS in all subpopulations assessed (see Table 2.2). When combined with the W4SS and used in parallel, whereby a positive screen for either tool led to a diagnostic test, it was found to have similar or higher sensitivity and specificity to the W4SS for all populations, depending on the cut-off threshold used and the subpopulation assessed. CRP was found to be most accurate among outpatients who were not on ART, compared with the W4SS alone, which had a sensitivity of 0.84 (95% CI: 0.75–0.90) and specificity of 0.37 (95% CI: 0.25–0.50) in this subpopulation. When performed sequentially after a positive W4SS among people living with HIV not on ART, CRP with a cut-off of > 5 mg/L was found to be as sensitive (0.84; 95% CI: 0.73–0.90) as the W4SS alone but to have significantly higher specificity (0.64; 95% CI: 0.55–0.72). Similar to the W4SS, the specificity of CRP for TB screening among inpatients living with HIV was found to be extremely low, likely due to other comorbidities that would also result in raised CRP levels and the presence of symptoms.
Table 2.2 Diagnostic accuracy of CRP using a cut-off of > 5 mg/L among different subpopulations of people living with HIV compared with culture as a reference standard
As a point-of-care biomedical test, CRP represents an opportunity for enhancing TB screening among people living with HIV. Health staff and patients might be more motivated to pursue a confirmatory diagnostic test following a positive screen for CRP. The specificity and predictive value of the test for detecting TB, however, will likely be reduced in settings with a lower TB prevalence than in those included in the meta-analysis.
Recommendation 4: Chest X-ray
Where available, WHO recommends using CXR in parallel with the W4SS, to assist in ruling out TB disease prior to initiating TPT among people living with HIV who are on ART. The GDG agreed that, due to the increased sensitivity, the evidence supported using CXR in addition to the W4SS as a parallel screening strategy in which a positive or abnormal result on either screen would indicate a referral for diagnostic evaluation. Data on “any abnormality” and an “abnormality suggestive of TB” detected by CXR were reviewed and either approach is recommended, depending on the context, the availability of radiological expertise, resources and preference towards higher sensitivity or higher specificity.
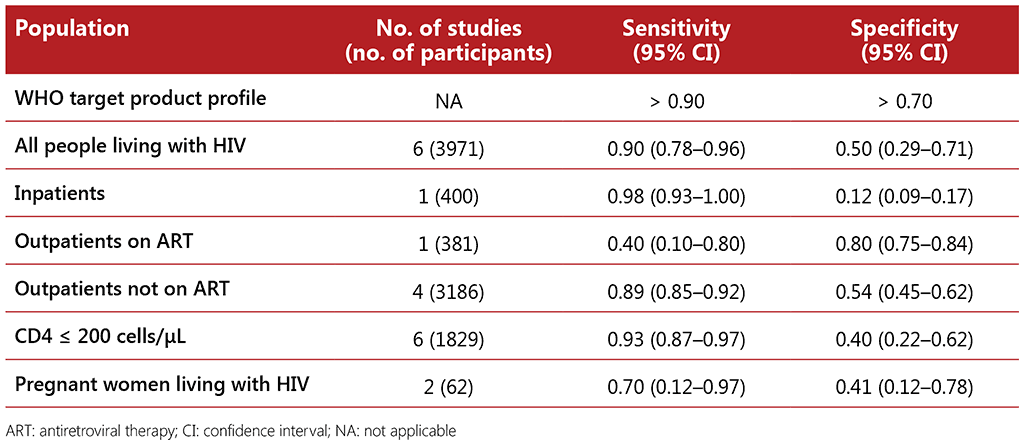
The evidence reviewed for the performance of CXR and the W4SS for all people living with HIV came from eight studies conducted in Benin, Botswana, Brazil, Guinea, India, Kenya, Malawi, Myanmar, Peru, South Africa and Zimbabwe, with a total of 6238 participants. The average prevalence of TB in all people living with HIV in the studies was 7%, ranging from 3% to 18%. Among outpatients on ART, the average prevalence was 2.6%.
CXR alone was found to have similar sensitivity to and similar or higher specificity than the W4SS across all subpopulations. When combined in a sequence whereby CXR followed a positive W4SS screen, CXR had a lower or similar sensitivity with higher or similar specificity. When combined and used in parallel with the W4SS, whereby a positive screen from either tool indicates the need for a diagnostic test, it had a higher or similar sensitivity and similar specificity (see Table 2.3). The IPD meta-analysis found this strategy to have the highest sensitivity (0.85; 95% CI: 0.69–0.94) compared with the W4SS (0.53; 95% CI: 0.36–0.69) and the other tools and strategies assessed for TB screening in outpatients on ART. While the data were limited for inpatients living with HIV, the combined strategy of CXR and the W4SS had a very low specificity (0.07; 95% CI: 0.03–0.19), similar to findings for using CRP or the W4SS alone.
Table 2.3 Diagnostic accuracy among different subpopulations of people living with HIV of the W4SS combined with CXR (any abnormality) compared with culture as the reference standard and using a positive or abnormal result on either screen or both
Recommendation 5: Computer-aided detection of chest X-ray
In many settings, the use of CXR for TB screening and triage for TB disease is limited by the unavailability of trained health personnel to interpret radiography images and by substantial intra-and inter-reader variability in its accuracy to detect abnormalities associated with TB. Numerous software packages that provide CAD, or automated interpretation of digital CXR images for the express purpose of determining the likelihood of TB disease, have been developed and offer a potential technological answer to the numerous implementation challenges inherent in human interpretation of CXRs.
For the development of the 2021 TB screening guidelines the performance of three CAD software programmes was compared with the performance of human readers. Due to methodological challenges, the estimates of CAD diagnostic accuracy were not able to be pooled across software programmes or across evaluations. Thus, the performances of CAD programmes and human readers from the included evaluations were presented as ranges (see Table 2.4).
Table 2.4 Sensitivity and specificity ranges of computer-aided detection software and human readers interpreting digital chest radiographs for detection of bacteriologically confirmed TB across three software programmes, from three independent evaluations of the software in a range of populations and settings
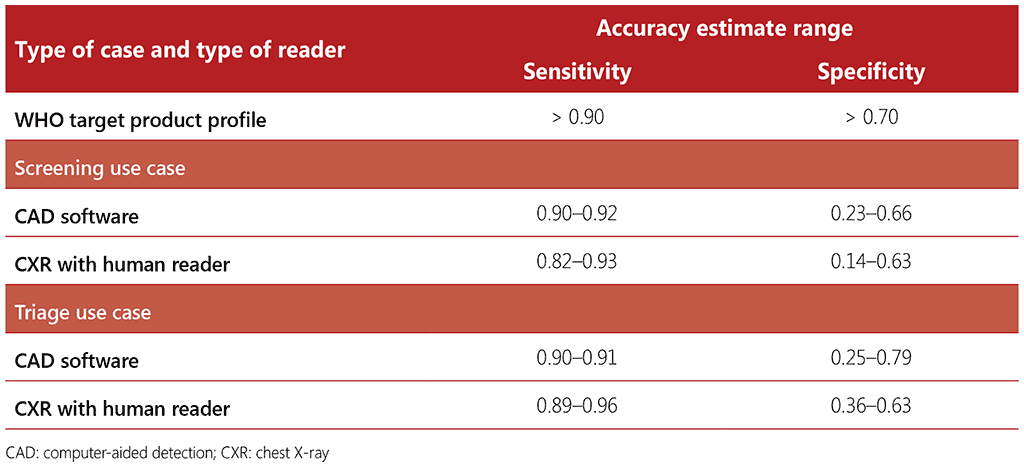
The results of the evaluation showed the variability of both human readers and CAD software programmes across different settings and populations. In comparing the range of accuracy of CAD to that of human readers interpreting CXRs and noting the variability of readers and the substantial overlap between the two ranges, the data suggested there is little difference between the two. Therefore, the GDG considered that CAD software programmes can be considered accurate when compared with human readers.
The recommendation applies to software brands that upon external validation demonstrate a performance that is not inferior to the products reviewed by the GDG in 2020. The analysis for this recommendation was restricted to bacteriologically confirmed TB and, thus, the recommendation may not necessarily apply to other forms of TB (such as exclusively extrapulmonary TB or clinically diagnosed TB).
This recommendation is specific to adults and adolescents aged 15 years and older but applies regardless of HIV status. Limited data were available for comparing CAD to human interpretation of CXR among people living with HIV; further evidence is needed about the performance of CAD software among people living with HIV, to enable better setting-specific and patient-specific calibration of CAD software.
Recommendation 6: Screening for TB using molecular WHO-recommended rapid diagnostic tests
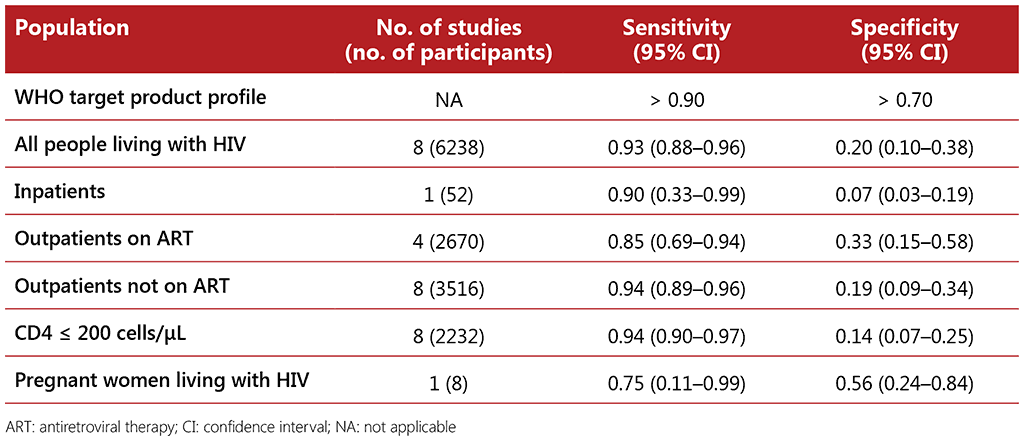
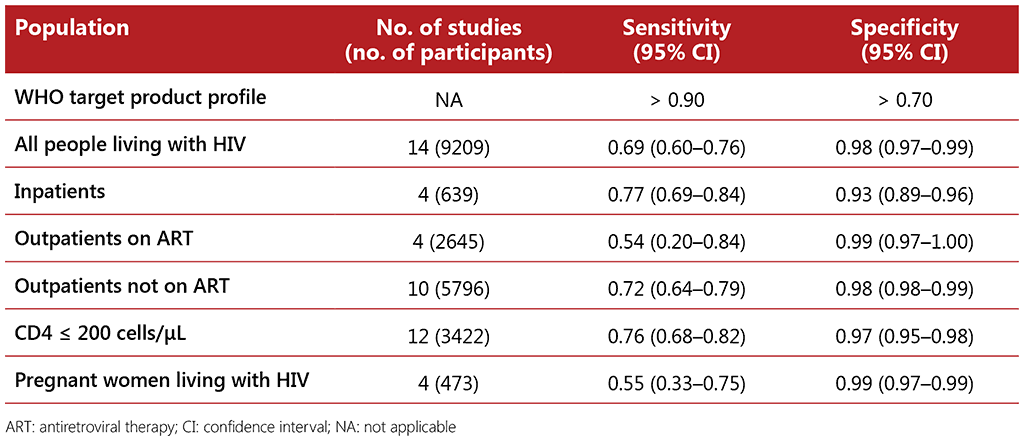
The systematic review of the performance of an mWRD used to screen for TB among people living with HIV included 14 studies with a total of 9209 participants. The Xpert MTB/RIF assay was the primary mWRD used in these studies. The prevalence of TB in the studies ranged from 1% to 26%. The average TB prevalence among participants attending outpatient facilities was 8.6%. Using an mWRD alone was found to have sensitivity of 0.69 (95% CI: 0.60–0.76) and specificity of 0.98 (95% CI: 0.97–0.99) compared with using the W4SS followed by an mWRD as a diagnostic test, which had sensitivity of 0.62 (95% CI: 0.56–0.69) and specificity of 0.99 (95% CI: 0.97–0.99) (see Table 2.5). There were no significant differences in the accuracy of the mWRD between the different subpopulations when compared with using the W4SS followed by the mWRD.
Due to the increased sensitivity of mWRDs, but also in consideration of the likely challenges relating to access, high costs and feasibility in many countries, mWRDs are recommended conditionally as an option for screening for TB disease among all adults and adolescents living with HIV, who are not medical inpatients in settings where the TB prevalence exceeds 10% (for whom there is a strong recommendation, see below). As with all screening tools, the GDG emphasized the importance in all settings of following up an mWRD screen with a diagnostic assessment (see Section 2.2) to prevent the potential harm of overtreatment. In addition, due consideration should be made to prioritizing mWRDs as a diagnostic test for all people with presumptive TB before scaling up mWRD as a screening test.
Table 2.5 Diagnostic accuracy of mWRD for screening for TB among different subpopulations of people living with HIV compared with microbiological reference standard
Recommendation 7: Screening for TB using molecular WHO-recommended rapid diagnostic tests among medical inpatients with HIV
TB is the main cause of hospitalization and mortality among people living with HIV (12). Given the high mortality among people living with HIV who are medical in-patients in TB high burden settings, WHO strongly recommends the use of mWRDs for rapid work-up in this population, regardless of symptoms. The assessment of the performance of an mWRD used as a combined TB screening and diagnostic strategy for medical ward patients with HIV included four studies in Ghana, Myanmar and South Africa with a total of 639 participants. The prevalence of TB in the included studies was 23.8%, ranging from 7% to 26%. The mWRD test assessed in the IPD was primarily the Xpert MTB/RIF assay.
Using the W4SS alone had 96% sensitivity and 11% specificity in the IPD meta-analysis of medical ward inpatients living with HIV, 94% of whom were positive on the W4SS. Thus, the difference in accuracy was minimal between the full screening and diagnostic strategy of using W4SS followed by mWRD, and using mWRD alone. Therefore, the value of the W4SS was judged to have limited utility in screening for TB in this population prior to an mWRD test, and the GDG recommended that medical inpatients should be screened and tested with an mWRD, irrespective of symptoms, to inform a decision about whether to treat for TB. A 10% threshold TB prevalence among hospital inpatients living with HIV is recommended, taking into account the TB prevalence among the participants studied and striking a balance between ensuring rapid diagnosis in this critically ill population and the need to avoid overtreatment. In lower prevalence settings, a screening and diagnostic strategy with mWRD alone would give rise to higher numbers of false positives, with overtreatment and the related social and economic consequences, including potential delay in starting ART. This recommendation may not be applicable to settings with a lower pre-test probability of TB.

 Feedback
Feedback