Book traversal links for 1266
A2.1 Introduction
Early identification, ongoing monitoring and proper management of adverse events (AEs) associated with anti-tuberculosis (TB) medications are crucial for relieving suffering and improving the quality of care for patients undergoing treatment for all forms of drug-resistant TB (DR-TB). Awareness and optimal management of AEs are essential parts of a holistic approach to supporting patients to adhere to various treatment regimens. A holistic approach requires comprehensive education and training for health care workers, and provision of appropriate information and counselling for patients and their treatment supporters. From the early stages of DR-TB treatment, patients and their care providers must be aware of the potential AEs associated with anti-TB drugs; how to prevent, manage or cope with common but non-serious AEs; how to recognize serious or severe signs and symptoms; and when and where to seek medical assistance or psychosocial support. Health care workers must be prepared to anticipate, prevent, monitor and manage common AEs, particularly those that may affect patients’ adherence to treatment; rapidly identify serious or potentially serious AEs and respond appropriately; record details of all AEs associated with DR-TB treatment; and know when to notify the responsible authorities.
National TB programmes (NTPs) should have established systems for active TB drug-safety monitoring and management (aDSM). Such systems involve the active and systematic clinical and laboratory assessment of patients being treated with new TB medicines or novel DR-TB regimens, with the aim of detecting, managing and reporting suspected or confirmed drug toxicities (1). The details of the aDSM framework are described in Annex 3.
A2.2 AEs and drug–drug interactions associated with DR-TB treatment
AEs associated with DR-TB treatment
Several systematic reviews have summarized AEs associated with multidrug-resistant or rifampicin-resistant TB (MDR/RR-TB) treatment over the past decade; however, most of these reviews could not assign causal relationships between specific anti-TB drugs and the reported AEs. Furthermore, most meta-analyses were conducted at a time when injectable drugs were commonly used in MDR/RR-TB treatment regimens. Other systematic reviews have focused on specific drugs and the AEs commonly associated with their use, but patients with MDR/RR-TB are usually treated with multidrug regimens; hence, attribution of causality can be difficult.
In 2020, the Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB Treatment used the individual patient data (IPD) MDR database (created for the meta-analysis of MDR-TB treatment and outcomes that was published in 2018) to obtain IPD from studies published between 2009 and 2016 on AEs that led to permanent discontinuation of anti-TB drugs (2). The group also obtained patient-level data that were shared with the World Health Organization (WHO) in response to a public call in 2018. Thus, the combined data from more than 9000 patients with MDR/RR-TB in 28 countries were used to conduct an IPD meta-analysis to estimate the frequency of AEs that led to permanent discontinuation of 20 different anti-TB drugs. The analysis did not include high-dose isoniazid, rifabutin, gatifloxacin or delamanid because too few patients received these drugs across the included studies. Almost one quarter of patients had at least one drug permanently stopped because of an AE, and stopping of one or more drugs was significantly more likely among female patients, older people and those receiving treatment in high-income countries. Fluoroquinolones, clofazimine and bedaquiline had the lowest incidence of AEs leading to permanent drug discontinuation, whereas second-line injectable drugs, aminosalicylic acid and linezolid had the highest incidence (2). Table A2.1 outlines the types of AEs associated with each of the key drugs in this analysis.
Table A2.1. Types of AEs resulting in permanent discontinuation of TB drugs
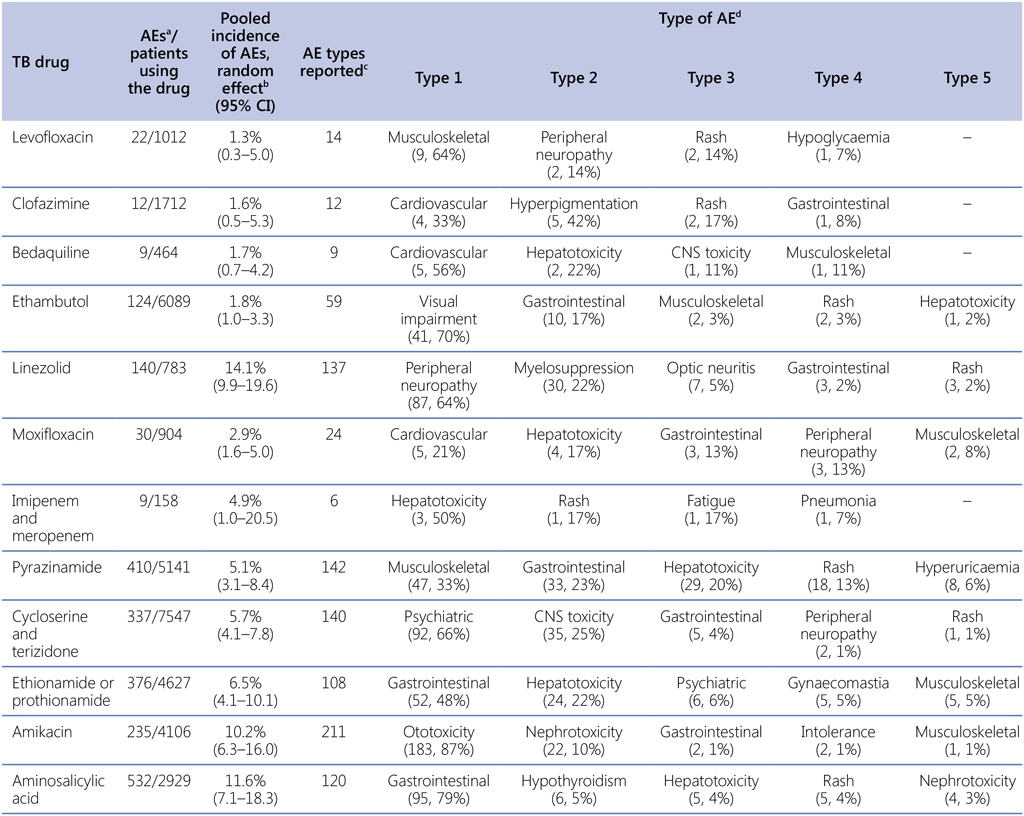
AE: adverse event; CI: confidence interval; CNS: central nervous system; TB: tuberculosis.
a AEs were defined as those that resulted in permanent discontinuation of a drug.
b Pooled incidence of AEs was estimated through meta-analysis of proportions.
c This analysis included only studies that reported AE types.
d For each drug, simple pooling was done to calculate the number of each type of AE; the five most common AE types with the corresponding proportions are presented.
Source: Table modified from Lan et al. (2020) (2).
In addition to the IPD-MDR analysis, the first global report from the WHO global aDSM database presented an interim analysis of safety data reported between July 2017 and August 2019; it covered 658 patients receiving regimens based on bedaquiline or delamanid (or both) for treatment of MDR/ RR-TB at participating centres in 26 countries (3). Because the data were prospectively collected, it was possible to assign causal attribution of AEs to specific drugs through external assessment of reported events, discussion with reporting clinicians and consideration of the scientific evidence available for each drug during the study period. The drugs most often used in treatment regimens for this cohort, in addition to bedaquiline or delamanid, were linezolid, moxifloxacin, levofloxacin, clofazimine, capreomycin, amikacin and carbapenems. Fig. A2.1 illustrates the distribution of reported serious AEs by organ or system.
Fig. A2.1. Summary of the distribution of 57 SAEs, by organ or system, among 658 patients treated for MDR/RR-TB in 26 countries between 2017 and 2019
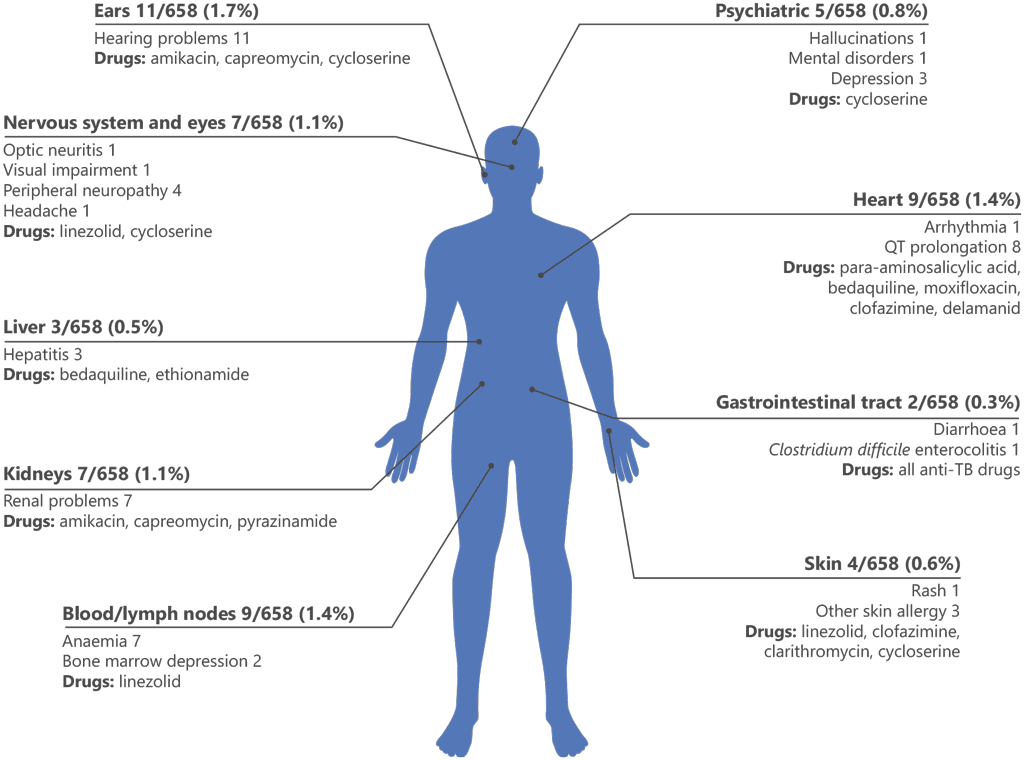
MDR/RR-TB: multidrug-resistant or rifampicin-resistant TB; SAE: serious adverse event; TB: tuberculosis.
Source: Borisov et al. (2019) (3).
Longer, individualized treatment regimens for MDR/RR-TB often contain many drugs with overlapping toxicities; hence, the frequency of AEs will depend on the combination of drugs in the regimen. The drug information sheets in Annex 1 outline clinically significant AEs associated with each of the WHO-recommended anti-TB drugs.
For the shorter regimens, the frequency of specific AEs may be more predictable because these regimens are standardized. The most common AEs associated with the 9-month all-oral regimens are anaemia (among patients receiving the linezolid-containing regimen), hepatotoxicity, QT prolongation, nausea and vomiting (4). The most common AEs for the BPaLM (bedaquiline, pretomanid, linezolid and moxifloxacin) regimen (e.g. peripheral neurophathy, anaemia, optic neuritis and hepatotoxicity) are mostly associated with linezolid. AEs associated with the BDLLfxC (bedaquiline, delamanid, linezolid, levofloxacin and clofazimine) regimen are also mostly attributed to linezolid, with anaemia being the most commonly reported serious AE in the Building Evidence to Advance Treatment of TB (BEAT) Tuberculosis trial in South Africa (5) and the BEAT-India study in India (6). In both of these studies, skin hyperpigmentation and acneiform rashes were the most commonly reported AEs among people receiving clofazimine-containing regimens.
Children generally experience fewer AEs from second-line TB treatment than adults, and most of the AEs are mild or moderate; however, treatment-related AEs appear to be more common among children who are HIV-positive (7). Furthermore, monitoring and timely detection of AEs (e.g. through repeated blood sampling, clinical assessment of vision and neuropathies, and identification of emergent neuropsychiatric events) can be more challenging in children than in adults.
Data on AEs among women who are pregnant or breastfeeding and receiving shorter standardized treatment regimens for DR-TB are relatively limited because pregnant women continue to be excluded from clinical TB research trials. However, many of the AEs often associated with MDR/RR-TB treatment, as outlined above (e.g. anaemia, peripheral neuropathy, nausea and vomiting), may be masked or exacerbated by physiological changes and common symptoms of pregnancy. Women who are pregnant or breastfeeding should continue to be monitored for the same AEs at least as frequently as non-pregnant patients receiving the same treatment (8).
Common drug–drug interactions associated with DR-TB treatment
Drug–drug interactions (DDIs) are common with many of the medications used in MDR-TB regimens, especially those involving bedaquiline. For each possible DDI, if the clinician determines that the potential benefits outweigh the risks (considering alternative treatment options), treatment may proceed with caution. Details of interactions for individual drugs used in all WHO-recommended TB regimens are provided in Annex 1.
Vigilance or, preferably, drug substitution should be considered when certain medications are prescribed concurrently with a DR-TB regimen; for example:
- TB treatments and antiretroviral therapy (ART) can have DDIs and overlapping toxicities, including these common ones:
- zidovudine and linezolid can lead to an increased risk of myelosuppression;
- boosted protease inhibitors can elevate bedaquiline levels;
- efavirenz can induce the metabolism of bedaquiline and thus lower bedaquiline concentrations (hence, alternative ART should be considered for patients who are prescribed efavirenz);
- linezolid is known to be associated with serotonin syndrome; therefore, caution should be taken with using other serotonergic drugs (e.g. sertraline and fluoxetine);
- concomitant drugs that prolong QT interval should be avoided if possible – such drugs require extra vigilance and monitoring with electrocardiography (ECG) if prescribed with bedaquiline and moxifloxacin; for example:
- ondansetron, methadone, amitriptyline, clarithromycin, and neuroleptics-phenothiazines (e.g. thioridazine, haloperidol, chlorpromazine, trifluoperazine, pericycline, prochlorperazine, fluphenazine, sertindole and pimozide);
- quinoline antimalarial drugs (e.g. halofantrine, chloroquine, hydroxychloroquine and quinacrine);
- anti-arrhythmic drugs (e.g. quinidine, procainamide, encainide, disopyramide, amiodarone, flecainide and sotalol);
- CYP3A4 inhibitors and CYP3A4 inducers can interact with bedaquiline:
- CYP3A4 inhibitors include the azole antifungal medications (ketoconazole, voriconazole and itraconazole), ketolides, such as telithromycin, and macrolide antibiotics other than azithromycin; in general, the azole antifungals can safely be used for less than 2 weeks, whereas fluconazole can potentially be used for more than 2 weeks;
- CYP3A4 inducers include phenytoin, carbamazepine, phenobarbital, St John’s wort (Hypericum perforatum), rifamycins and glucocorticoids;
- drugs inducing myelosuppression (e.g. azathioprine, cytotoxic agents and zidovudine) should be used with caution; and
- DDIs between DR-TB and drugs for hepatitis C are detailed in Chapter 2, Section 8.4.
A2.3 Monitoring AEs of interest associated with DR-TB treatment
Treatment monitoring schedules must include relevant clinical and laboratory parameters to detect, manage and prevent common and serious AEs in a timely manner (Table A2.2). Many AEs are easy to recognize (e.g. skin hyperpigmentation due to clofazimine), and most adults, adolescents and older children can largely describe the symptoms they experience. However, some patients or caregivers may be reticent about reporting symptoms or might only remember them when asked about specific problems; thus, it is important to have a systematic approach when screening for potential AEs. Safety monitoring requirements will vary depending on the chosen treatment regimen, because some parameters apply to specific drugs (e.g. complete blood counts [CBCs] during exposure to linezolid), whereas others (e.g. liver function tests [LFTs]) measure AEs associated with a wider variety of anti-TB drugs. The frequency of evaluation of specific safety monitoring parameters for various treatment regimens is outlined in Table A2.2. Further information on key monitoring parameters is given below.
Brief peripheral neuropathy screen
All patients should be assessed every 2 weeks for a month, then monthly, for symptoms suggesting neuropathic pain, using the brief peripheral neuropathy screen (BPNS), which allows for subjective grading of the symptoms. Although neuropathic pain is difficult to define and varies from one person to another, it is often described as “burning”, “electric”, “tingling” and “shooting” in nature, and can vary from a constant pain to intermittent sharp, shooting pains. As described, the pain is most often present without associated stimulation, but it can be exacerbated by stimuli. The BPNS is a non-invasive, fast, cheap, easy-to-do diagnostic clinical tool that was developed by the AIDS Clinical Trials Group (9).
Other common causes of peripheral neuropathy include alcohol use, certain other medications, HIV infection, pregnancy and diabetes, and these causes should also be assessed in any patient found to have peripheral neuropathy while receiving DR-TB treatment.
For a definitive diagnosis of peripheral neuropathy, patients with symptoms should be examined, including deep tendon ankle reflexes, light touch perception with a cotton swab or monofilament, or vibration testing with a 128-Hz tuning fork.
Haematological assessment
Because of the risk of myelosuppression associated with even relatively short exposures to linezolid, pretreatment assessment of haemoglobin (Hb), neutrophil and platelet levels is crucial for patients considering treatment with a linezolid-containing regimen. Severe anaemia in patients with TB is a significant risk factor for poor treatment outcomes (10), and patients with a low baseline Hb level may be at higher risk of severe linezolid-induced haematological toxicity (11). Linezolid should be administered with caution to patients with a pretreatment serum Hb level below 80 g/L that cannot be rapidly corrected (i.e. with a blood transfusion) before starting DR-TB treatment. Similarly, owing to the morbidity associated with severe neutropenia and thrombocytopenia, treatment with linezolid should be administered with caution to patients with neutrophil levels below 0.75 × 10⁹/L or platelet levels below 50 × 10⁹/L before starting treatment. Owing to the high risk of anaemia posed by linezolid, even among people with normal blood counts before starting treatment, Hb level must be checked every 2 weeks at least for the first month, then monthly for the duration of linezolid exposure. CBCs should be performed more often if clinically indicated (i.e. if there are signs and symptoms of myelosuppression, particularly if levels of Hb, neutrophils or platelets were relatively low at the start of treatment).
Other causes of anaemia (e.g. iron deficiency, other nutritional deficiencies or occult gastrointestinal bleeding) should be considered and appropriately managed, although these are less likely to occur in the middle of treatment, especially if the patient’s condition is improving. Additional work-up and management of anaemia involve the following:
- review of CBC, including the red blood cell (RBC) indices, white cell differential counts and platelet levels, and other investigations to determine possible etiology of anaemia;
- review of other medications that could possibly be associated with anaemia; and
- assessment for other infections, including parasites and viral pathogens (i.e. parvovirus).
The RBC indices, including the mean corpuscular volume of the CBC, can be used to assess whether the anaemia is microcytic or macrocytic. Microcytic anaemia is often caused by iron deficiency, for which iron supplements are indicated; macrocytic anaemia is usually caused by vitamin B12 or folate deficiency.
Visual acuity and colour vision tests
The first sign of optic neuritis is usually the loss of red–green colour distinction. This is best tested using the Ishihara test for colour vision (12). Optic neuritis can also manifest as decreased visual acuity, which can be tested using the Snellen chart (13). These tests should be undertaken every 2 weeks for 1 month after starting a linezolid-containing regimen, then monthly until the end of treatment. Results should be compared with the baseline or previous test results to establish a change in colour vision or visual acuity.
LFTs
It is advisable to screen for viral hepatitis B and C in patients who are due to receive regimens with multiple hepatotoxic drugs (e.g. BPaLM or BPaL [bedaquiline, pretomanid and linezolid]), because these patients require monthly liver function monitoring throughout treatment. All patients starting treatment for DR-TB must have serum liver enzyme levels (at least alanine aminotransferase [ALT]) checked at baseline, and liver enzyme tests should be repeated for all patients who experience signs and symptoms of hepatotoxicity through treatment.
QT interval monitoring
The absolute QT interval is measured from the onset of the Q-wave to the termination of the T-wave. Either limb lead II or precordial lead V5 is best for measuring the QT interval; alternative leads may be used if T-wave termination is poorly visualized. The normal QT interval changes physiologically (e.g. depending on time of day, level of activity and emotional state). Several formulae may be used to correct the absolute QT interval for an individual’s heart rate (11). The Fridericia correction (QTcF) measures the QT interval in milliseconds divided by the cubed root of the RR (interval between two consecutive R waves) in seconds; a corrected QT interval between 450 ms and 500 ms is considered borderline, and a QTcF of more than 500 ms in adults or more than 450 ms in children warrants closer ECG monitoring. A QTcF of more than 500 ms would be an indication for interrupting QT-prolonging drugs (see Table A2.3).
Other factors that may trigger a requirement for closer ECG monitoring are symptoms of palpitations, dizziness, chest pain and syncope; however, these are not in themselves reliable indicators of increased risk of torsades de pointes (TdP) – a specific type of abnormal heart rhythm that can lead to sudden death. A corrected QT interval of more than 500 ms is considered dangerous and increases the risk of TdP with possible arrhythmic death. This level of QT prolongation typically occurs when multiple QT-prolonging drugs are used, particularly in individuals with additional risk factors, such as cardiac disease, hypokalaemia, hypomagnesaemia, hypocalcaemia or hypothyroidism.
Neuropsychiatric assessment
Several TB medications (e.g. cycloserine or terizidone, delamanid and high-dose isoniazid) are known to be associated with neuropsychiatric AEs, whereas other TB medications (e.g. clofazimine) have side-effects that may be socially unacceptable or stigmatizing in some settings. Furthermore, some patients may experience depression, anxiety or even psychosis during DR-TB treatment that is not directly related to specific drug AEs but may be due to underlying psychiatric disorders or other psychosocial stressors and catastrophic costs associated with the diagnosis and treatment of DR-TB. Additionally, the consumption of alcohol or other psychotropic substances can complicate the neuropsychiatric presentation. Screening for unsafe substance use should be performed using a validated tool, such as the ASSIST-linked brief intervention for hazardous and harmful substance use (14). The psychosocial aspects of DR-TB must not be ignored because they may have a considerable impact on patients’ mental health and adherence to treatment.
The nine-question Patient Health Questionnaire (PHQ-9) is a validated tool that was developed to assess the degree of depression in an individual (15). Ideally, PHQ-9 is done at baseline and then monthly, especially when cycloserine or high-dose isoniazid (or both) are part of the DR-TB regimen, or ad hoc when the patient is showing signs of depression.
Delamanid is reported to cause neuropsychiatric adverse effects, particularly in children (16). The most frequent are sleep disturbance and vivid nightmares, but insomnia, hallucinations and others have been reported. It is important to provide anticipatory guidance to patients and their families, so they can monitor for these adverse effects and follow-up with their health care providers if these occur. Most of these events are mild and self-limited, resolving without any intervention or change to the delamanid. If the events are not disruptive to the child or family, then continuing delamanid and monitoring is appropriate. If they are impacting the child and family more substantially, are persistent, or are more severe, then it is reasonable to temporarily hold the delamanid and observe. The events appear to resolve quickly after discontinuation. For mild-to-moderate events, re-introduction of delamanid after resolution can be done; often there will not be recurrence. If the event was more severe initially or recurs after reintroduction and remains disturbing to the child or family, then the delamanid should be permanently discontinued, although this is not commonly needed. It is important to consider other causes of these events as well, including central nervous system TB or other TB drugs like cycloserine or terizidone.
Signal of hallucinations in children treated with delamanid was reported and evaluated by the WHO advisory committee on safety of medicinal products, which suggested that the evidence of hallucinations in children is uncertain as the potential of misclassifying ‘night terrors’ and ‘nightmares’, which are common in child development, as ‘hallucinations’ (17). Given that the product label of delamanid has been modified by the market authorization holder to include hallucinations as an adverse event, further monitoring and assessment are required.
Renal assessment
It is important to assess kidney function at baseline to determine whether a patient requires renal dosing of selected anti-TB drugs (e.g. levofloxacin, cycloserine or terizidone, pyrazinamide, ethambutol, carbapenems, para-aminosalicylic acid and amikacin), if used. Patients with a history of renal disease (including comorbidities such as HIV and diabetes), advanced age or any renal symptoms, or with a low baseline glomerular filtration rate, should be monitored monthly or more often as necessary during treatment. If baseline renal function is normal, re-assessment of renal function during DR-TB treatment is only required if clinically indicated or if the patient starts taking nephrotoxic drugs, such as tenofovir.
Thyroid function assessment
Para-aminosalicylic acid or ethionamide/prothionamide (or both) can cause hypothyroidism, which may be suspected during clinical assessment and should be confirmed by testing levels of serum thyroid-stimulating hormone (TSH). Goitres can develop owing to the toxic drug effects, but symptoms can often be subtle and may be masked by TB symptoms or other AEs; hence, it is recommended that patients be screened for hypothyroidism (via serum TSH levels) every 3 months, or sooner if symptoms arise, for the duration of exposure to either of these drugs. Dosing of thyroid replacement therapy should be guided by serum TSH levels every month until a stable dose of the thyroid replacement hormone is reached, at which point 3-monthly monitoring may resume. In areas where iodine deficiency goitres are endemic, treatment with iodine is indicated, in addition to assessment and treatment for hypothyroidism.
Table A2.2. Recommended schedule of baseline, routine and post-treatment monitoring examinations and tests for patients receiving DR-TB treatment (applicable to all recommended MDR/RR-TB treatment regimens)
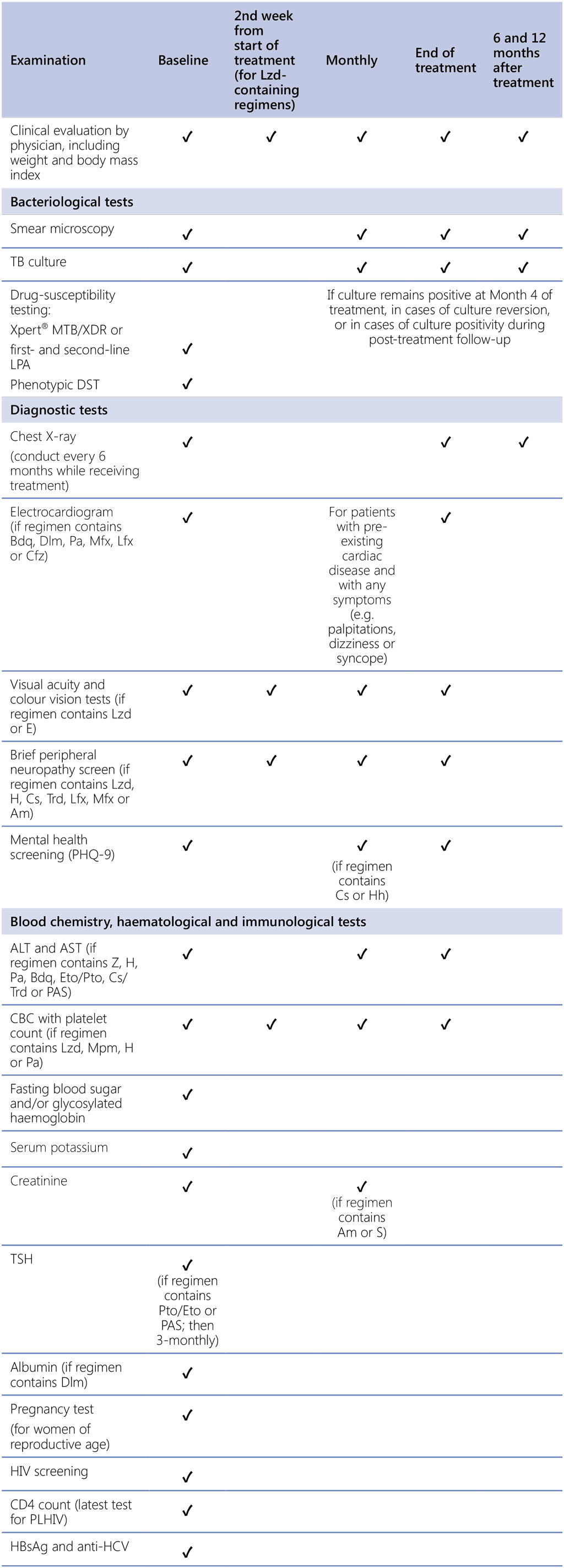
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CBC: complete blood count; DR-TB: drug-resistant TB; DST: drug-susceptibility testing; HbsAg: hepatitis B surface antigen; HCV: hepatitis C virus; HIV: human immunodeficiency virus; LPA: line probe assay; MDR/RR-TB: multidrug-resistant and rifampicin-resistant TB; PHQ-9: nine-question Patient Health Questionnaire; PLHIV: people living with HIV; TB: tuberculosis; TSH: thyroid-stimulating hormone.
Drugs: Am: amikacin; Bdq: bedaquiline; Cfz: clofazimine; Cs: cycloserine; Dlm: delamanid; E: ethambutol; Eto: ethionamide; H: isoniazid; Hh: high-dose isoniazid; Lfx: levofloxacin; Lzd: linezolid; Mfx: moxifloxacin; Mpm: meropenem; Pa: pretomanid; PAS: para-aminosalicylic acid; Pto: prothionamide; S: streptomycin; Trd: terizidone; Z: pyrazinamide.
A2.4 Management of AEs associated with DR-TB treatment
Most patients receiving DR-TB treatment experience one or more AEs associated with the TB medications. Therefore, ongoing education of patients and their caregivers or family members is crucial, to empower patients to anticipate and prepare for common AEs, and to be aware of signs and symptoms that may be serious and require urgent medical attention. Too much information at the start of treatment, especially just after receiving the diagnosis of DR-TB (a potentially life-threatening disease that the patient may not have heard of), can be overwhelming for the patient; therefore, information should be shared with as much detail and as often as the patient can cope with, in ways that the patient can understand. Some people may request information leaflets to read in their own time, or referral to relevant websites and other sources of accurate information; others may prefer to receive the information through individual counselling sessions or patient support groups, where they can also learn helpful coping strategies.
Acknowledgement of a patient’s experiences and reassurance by the health care provider is sometimes all that the patient needs to cope with the AEs associated with treatment, particularly if the patient understands the importance of completing a full course of effective treatment. Patients who feel negated, belittled or unheard may decide to stop taking their TB medications, particularly if the AEs outweigh the perceived benefit of their treatment (e.g. as they start feeling better and TB symptoms resolve). Some AEs may disappear or diminish over time, even without intervention, and patients may be able to continue taking their medication if they are sufficiently motivated to tolerate the non-serious AEs. Some AEs (e.g. skin hyperpigmentation) may be completely unacceptable to some patients, despite health care providers not considering the AE to be clinically serious enough to withhold the responsible agent from an otherwise effective treatment regimen. Additional medications to treat AEs can add to patients’ already heavy pill burden, and these ancillary medications come with their own side-effects. Clinicians must take patients’ preferences into account and, wherever possible, involve patients or their caregivers in decisions about whether to treat AEs with additional medications and when to withdraw TB medications in response to AEs, particularly if this might compromise the efficacy of their treatment regimen.
AE management strategies
Table A2.3 summarizes the common AEs associated with DR-TB treatment, the TB medications likely to be responsible for those AEs, suggested management strategies and other potentially useful information. In general, clinicians should avoid lowering doses of TB medications to reduce the incidence or severity of AEs. It may be possible to split doses or change the frequency of some drugs; however, this needs to be considered carefully for regimens based on BPaL. In the case of linezolid specifically, dose reduction, interruption and discontinuation, if absolutely necessary, may be done at certain points in the treatment course (see Chapter 2, Section 4 for further details). It is sometimes helpful to change the timing of administration of certain drugs, to help patients to cope with certain AEs, especially in relation to food intake and sleeping. Weight-banded dosing guidelines may allow for some medications (e.g. terizidone or cycloserine, ethionamide/prothionamide, pyrazinamide and ethambutol) to be administered at the lower end of the recommended dose range for people in a specific weight band. Pharmacokinetic studies of TB medications are helpful in determining an acceptable dosing range and frequency that balance safety and tolerability without compromising efficacy.
Overlapping toxicities between TB medications and ART drugs must be considered for patients who are coinfected with HIV; for example, efavirenz, as a CYP4A inducer, could lower levels of bedaquiline and pretomanid, and thus should not be given together with these anti-TB drugs. Also, zidovudine should be avoided in patients receiving linezolid because of the increased risk of myelosuppression.
Table A2.3. AEs, suspected agents and management strategies
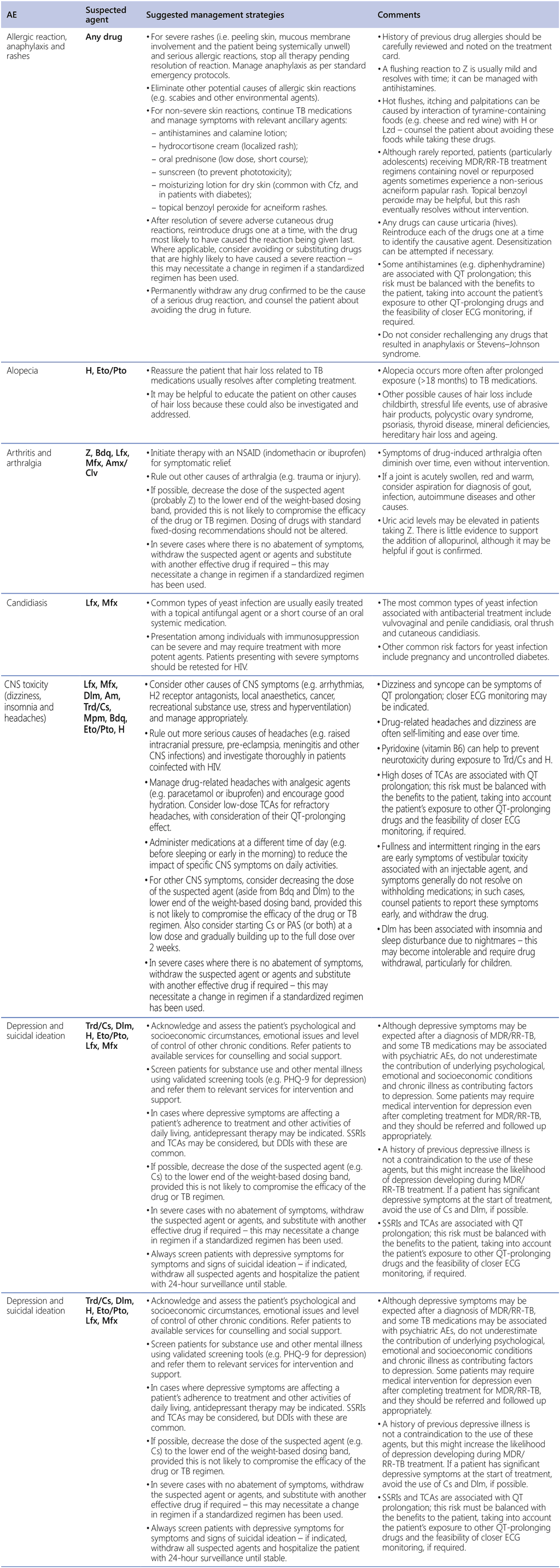
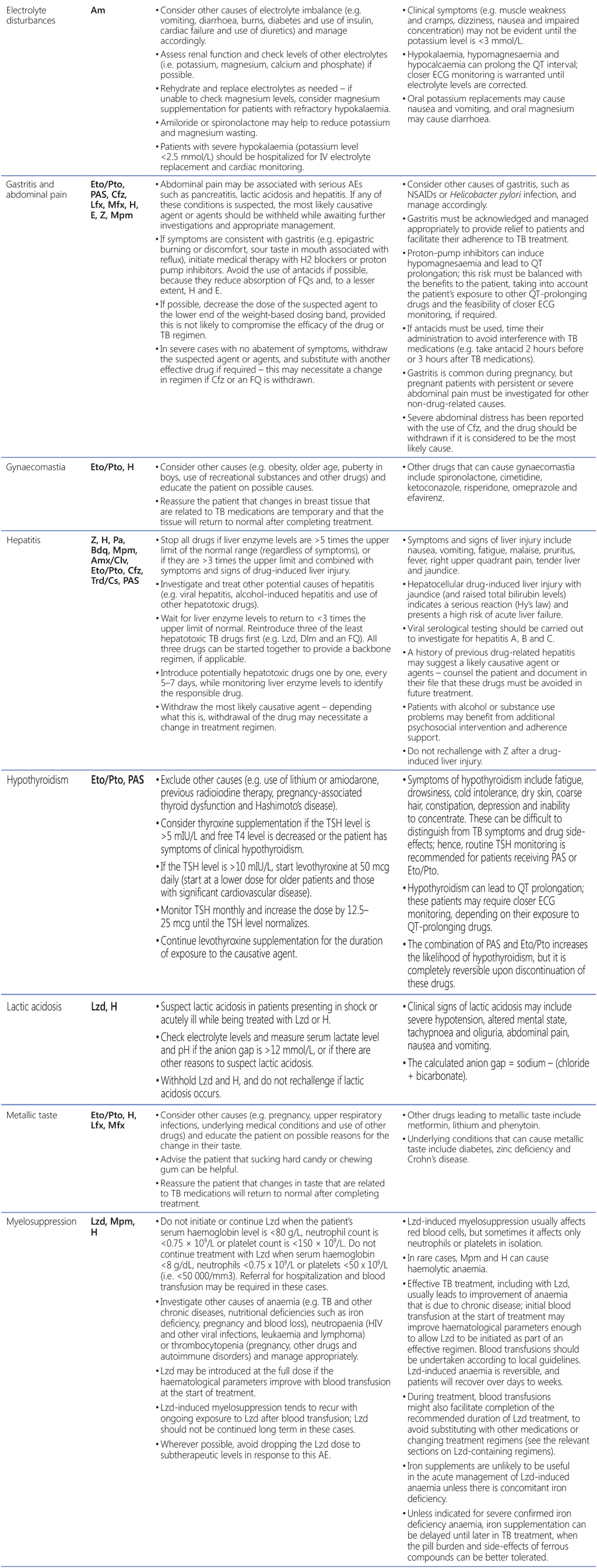
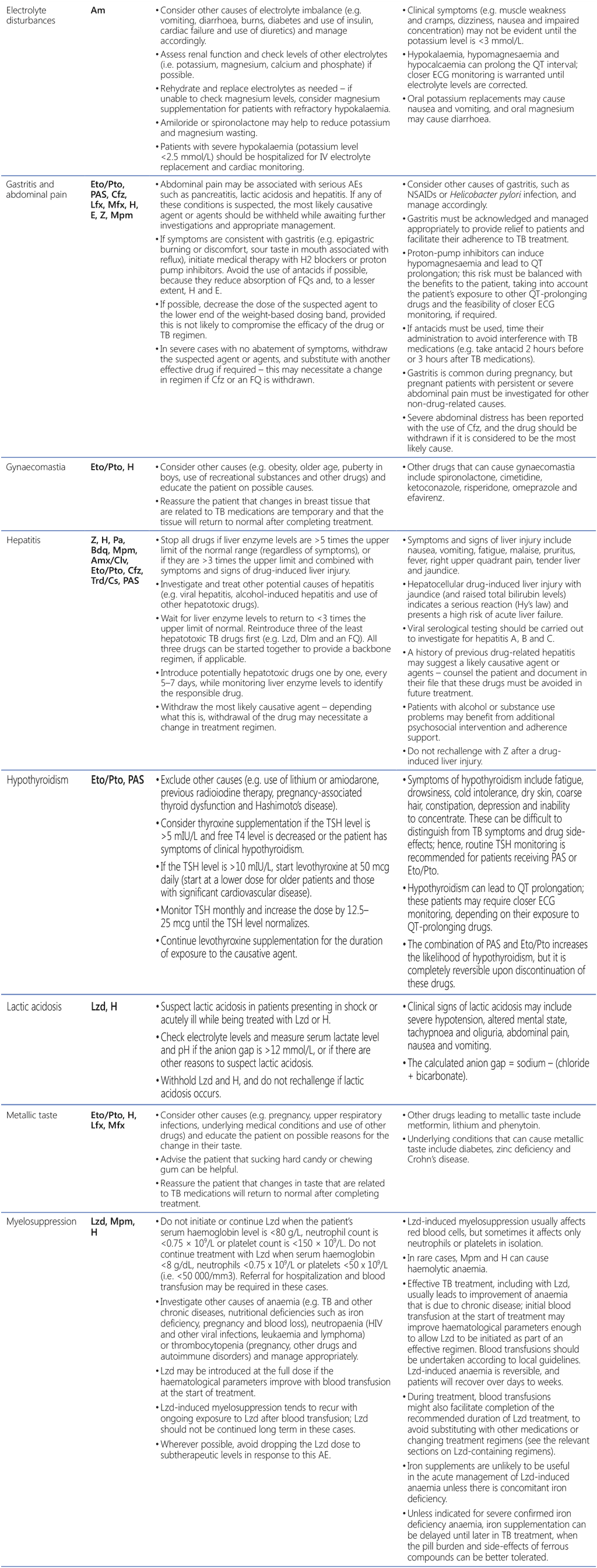
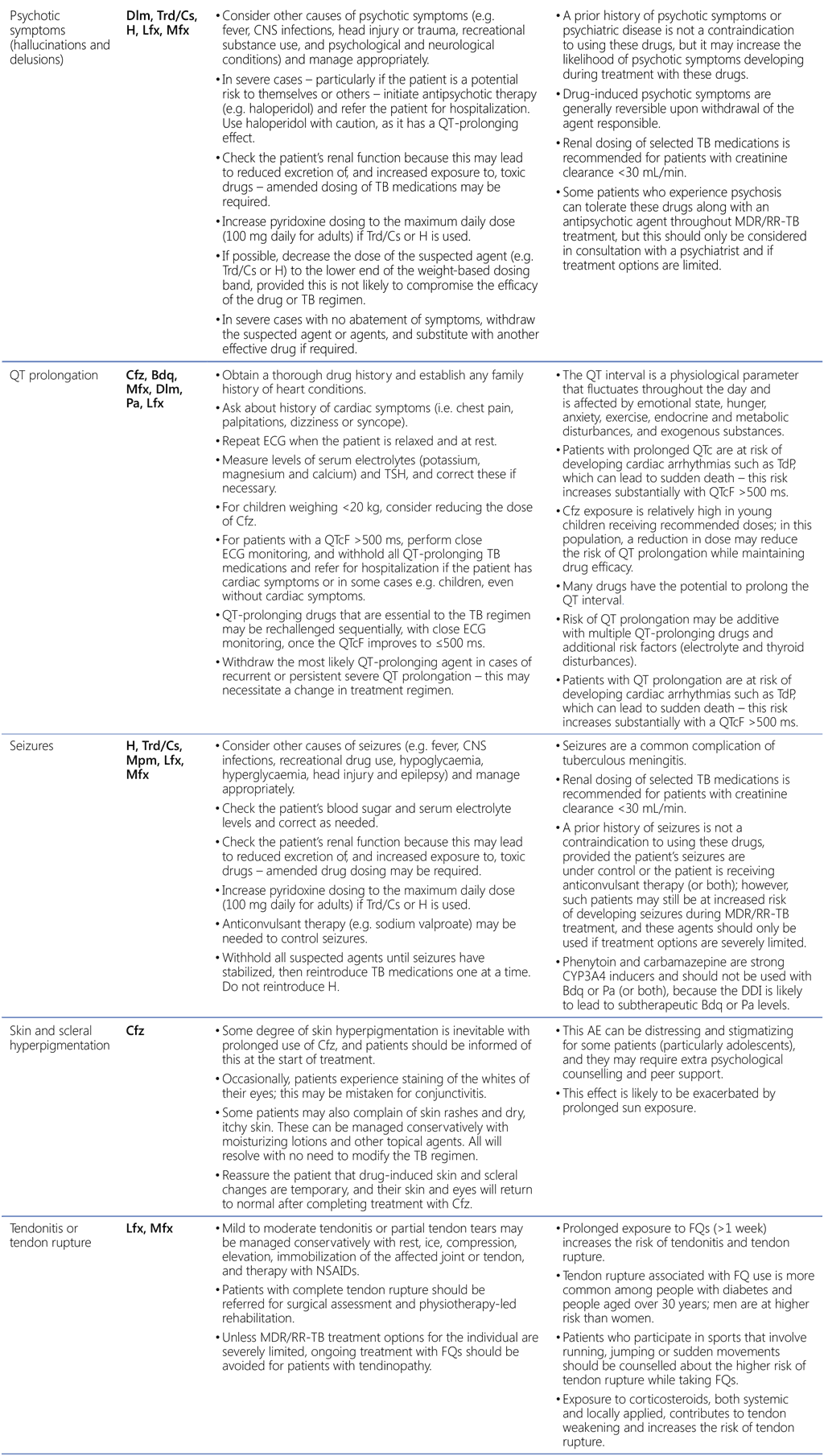
AE: adverse event; CNS: central nervous system; DDI: drug–drug interaction; ECG: electrocardiography; FQ: fluoroquinolone; H2: histamine type 2; HIV: human immunodeficiency virus; IU: international units; IV: intravenous; MDR/RR-TB: multidrug-resistant and rifampicin-resistant TB; NSAID: non-steroidal anti-inflammatory drug; PHQ-9: nine-question Patient Health Questionnaire; QTcF: corrected QT interval per Fridericia’s formula; SSRI: selective serotonin reuptake inhibitor; TB: tuberculosis; TCA: tricyclic antidepressant; TdP: torsades de pointes; TSH: thyroid-stimulating hormone.
Drugs: Am: amikacin; Amx: amoxicillin; Bdq: bedaquiline; Cfz: clofazimine; Clv: clavulanic acid; Cs: cycloserine; Dlm: delamanid; E: ethambutol; Eto: ethionamide; H: isoniazid; Lfx: levofloxacin; Lzd: linezolid; Mfx: moxifloxacin; Mpm: meropenem; Pa: pretomanid; PAS: para-aminosalicylic acid; Pto: prothionamide; Trd: terizidone; Z: pyrazinamide.
Ancillary medications
Clinical management of people receiving treatment for DR-TB often requires the use of ancillary medications to prevent, lessen or eliminate the AEs associated with TB medications. NTPs may choose to make ancillary medications available for health care providers to prescribe to patients free of charge, to reduce the burden of catastrophic costs on patients and their households. Table A2.4 presents a list of commonly used ancillary medications and their indications for use; this list may be adapted by countries according to best practices in their settings.
Table A2.4. Commonly used ancillary medications for treating AEs associated with TB medications
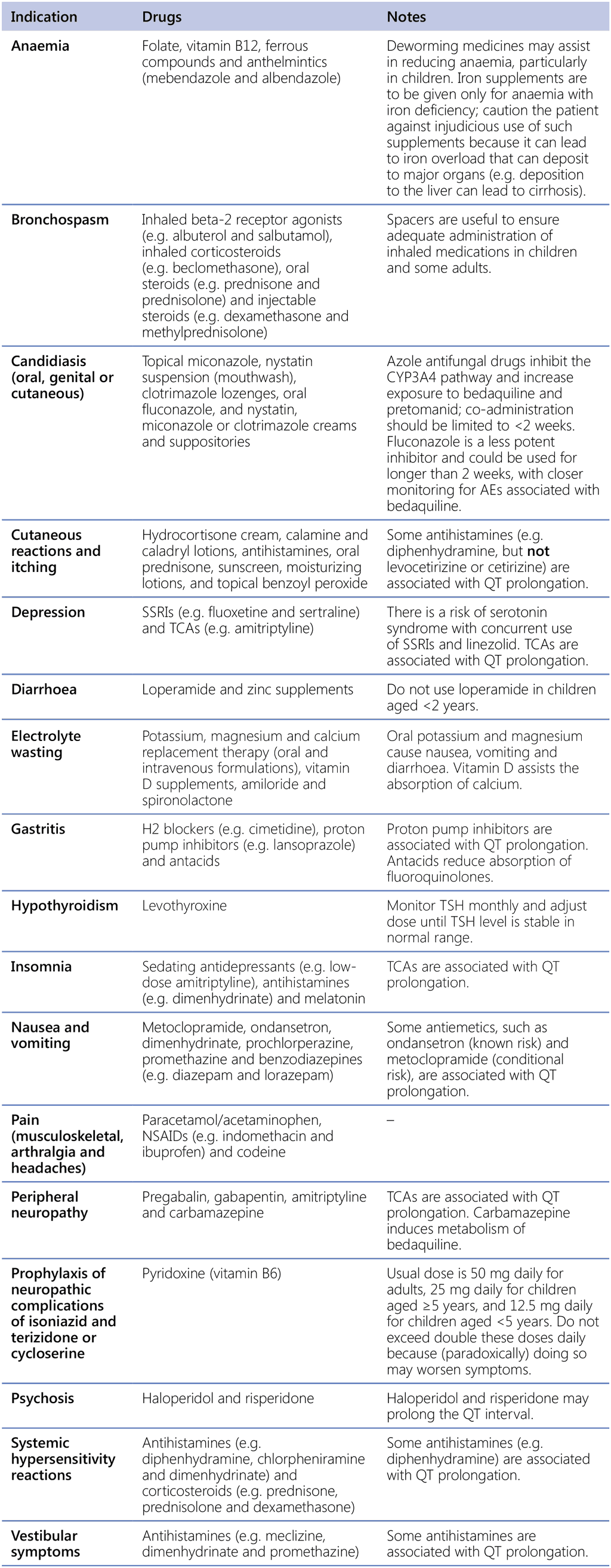
AE: adverse event; H2: histamine type 2; NSAID: non-steroidal anti-inflammatory drug; SSRI: selective serotonin reuptake inhibitor; TB: tuberculosis; TCA: tricyclic antidepressant; TSH: thyroid-stimulating hormone.
Management of AEs associated with the BPaLM regimen
Among 109 participants enrolled in the Nix-TB trial who received the BPaL (1200 mg/day) regimen, 57% experienced a Grade 3 or 4 AE and 17% experienced serious AEs. More than 80% of all participants experienced peripheral neuropathy, with most reporting mild or moderate symptoms, and 37% had anaemia (22). Twelve participants (11%) had an elevation in ALT level, and 11 (10%) had an elevation in AST to a level three times higher than the upper limit of normal (ULN); no participants had QT prolongation beyond 480 ms (22). In the ZeNix study, peripheral neuropathy of Grade 3 or lower was reported in 11 of 45 participants (24%) in the group that had received 600 mg of linezolid for 26 weeks. Laboratory-confirmed myelosuppression was reported in one of the 45 participants (2%) who had received 600 mg of linezolid for 26 weeks. Across the treatment groups, 47 of 181 participants (26%) had one or more liver-related AEs, with similar numbers in each group (23). In TB-PRACTECAL, 17% (26/151) of the BPaLM group experienced Grade 3 or higher AEs or serious AEs during or within 30 days after treatment, compared with 47% (71/151) in the standard of care group (24).
In a multicountry operational research study using the BPaL regimen,² 32 linezolid-associated AEs of special interest (AESI) occurred in the cohort of 53 patients using a linezolid dose of 600 mg/day, and two of these AESI (6.3%) were classified as severity Grade 3 or 4. In the group of 297 patients using a linezolid dose of 1200 mg/day, 308 AESI occurred; among them, 79 (25.6%) were classified as severity Grade 3 or 4: peripheral neuropathy (39/79), myelosuppression (38/79) or optic neuritis (2/79). In the LIFT-TB initiative, the rate of serious AEs reported among patients treated with BPaL was 24% (n=323) (25).
Regardless of AEs, more than 90% of participants in the Nix-TB, ZeNix and TB-PRACTECAL trials were able to complete more than 75% of the maximal intended dose and duration of linezolid (22). In the LIFT-TB initiative, 46% of patients receiving the BPaL regimen (148/323) experienced AEs that led to treatment modification (25). Among patients using linezolid 600 mg/day, the rate of permanent discontinuation of linezolid owing to peripheral neuropathy or optic neuritis was 7.5% (4/53). In the group of patients using linezolid 1200 mg/day (n=297), 42 patients (14%) had to permanently discontinue linezolid owing to peripheral neuropathy (30/42), optic neuritis (7/42) or myelosuppression (5/42).²
Table A2.5 provides general definitions of the severity of AEs from Grade 1 to Grade 4. Table A2.6 suggests how AEs associated with the BPaLM regimen could be managed, according to their severity grading.
Peripheral neuropathy
Peripheral neuropathy is extremely common in patients taking linezolid 1200 mg/day, and it was experienced by 81% of the patients in the Nix-TB study. The ZeNix and TB-PRACTECAL trials, which used the BPaLM or BPaL regimen with lower doses or a shorter duration of linezolid than 1200 mg for 6 months, showed high efficacy and improved safety, including in relation to peripheral neuropathy. In the ZeNix study, peripheral neuropathy occurred in 38% of those given linezolid 1200 mg/day and in 24% of those given linezolid 600 mg/day.
In the BPaL operational research study, 32.1% (17/53) of individuals receiving linezolid 600 mg developed peripheral neuropathy, with none being of Grade 3 or higher. In the linezolid 1200 mg group, 58.6% (174/297) of the patients experienced peripheral neuropathy, of which 22.4% (39/174) were Grade 3 or higher.²
Peripheral neuropathy generally occurs around 3 months after treatment initiation , with onset occurring after several weeks of drug exposure, depending on both the dose and duration of treatment with linezolid. Among patients who develop a mean score on the BPNS scale indicating moderate to severe neuropathy, the median time to return to a score indicating no or mild neuropathy was 3 months. After dose reduction, interruption or discontinuation of linezolid, the symptoms may disappear or significantly improve. However, in some cases, discomfort persisted, highlighting the importance of careful monitoring throughout treatment.
Myelosuppression
In the NiX-TB study, where linezolid was dosed at 1200 mg/day, myelosuppression occurred in 52 patients (48%); among the 40 of these patients (37% of the study population) who had anaemia, seven had decreases in Hb level to less than 80 g/L. In most of the patients who experienced this AE, it occurred during the first 2 months of treatment, with results being similar in HIV-coinfected and uninfected patients, and in patients who received different starting doses of linezolid. In the ZeNix study, myelosuppression occurred in 22% of those given linezolid 1200 mg/day versus 2% of those given 600 mg/day.
In the BPaL operational research study, 13 patients (24.53%) receiving linezolid 600 mg/day experienced myelosuppression, with none being of Grade 3 or higher. In the linezolid 1200 mg group, 125 patients (42.1%) experienced myelosuppression, 38 (30.4%) of which were Grade 3 or higher. Myelosuppression generally occurred within the first 3 months of treatment and was resolved in most of the patients with this AE.³
Optic neuritis
Among all the TB drugs, linezolid is the most common cause of optic neuritis, with the condition mostly developing after 3 months of treatment. Patients may develop painless, progressive, bilateral, symmetrical visual disturbances.
In the Nix-TB study, optic nerve disorders were reported in 11% of patients, with most AEs being of Grade 1 or Grade 2. Two cases were reported as serious AEs with confirmed optic neuritis or neuropathy; both cases resolved after discontinuation of linezolid, with visual acuity returning to baseline levels. In comparison, in the ZeNix trial, optic neuritis occurred in four patients (9%) receiving linezolid 1200 mg/day and none in the group receiving 600 mg/day. All four cases resolved after withdrawal of linezolid.
In the operational research study, optic neuritis was reported in 11 individuals from the cohort of 350 receiving the BPaL regimen. In the linezolid 600 mg daily group, two of 53 patients (3.8%) developed optic neuritis, and both cases were resolved or improved by the end of treatment. In the linezolid 1200 mg daily group, nine of 297 patients (3%) experienced optic neuritis, which had resolved or improved by the end of treatment for all of them, including two cases that resolved with sequelae.³
Hepatotoxicity
In patients receiving the BPaLM regimen, drug-induced liver injury can result from pretomanid or bedaquiline, and less often from other drugs. Chronic liver disease, secondary to alcoholic liver disease or infection with hepatitis B or C virus, increases the risk of drug-induced liver injury among patients receiving treatment for DR-TB (26).
QT prolongation
In patients receiving the BPaLM regimen, bedaquiline and moxifloxacin are known to be associated with prolonged QT intervals, as recorded by ECG.⁴ However, QT prolongation of Grade 3 or higher is rarely reported among patients receiving the BPaLM or BPaL regimen.
Table A2.5. General definitions of severity scale of adverse events
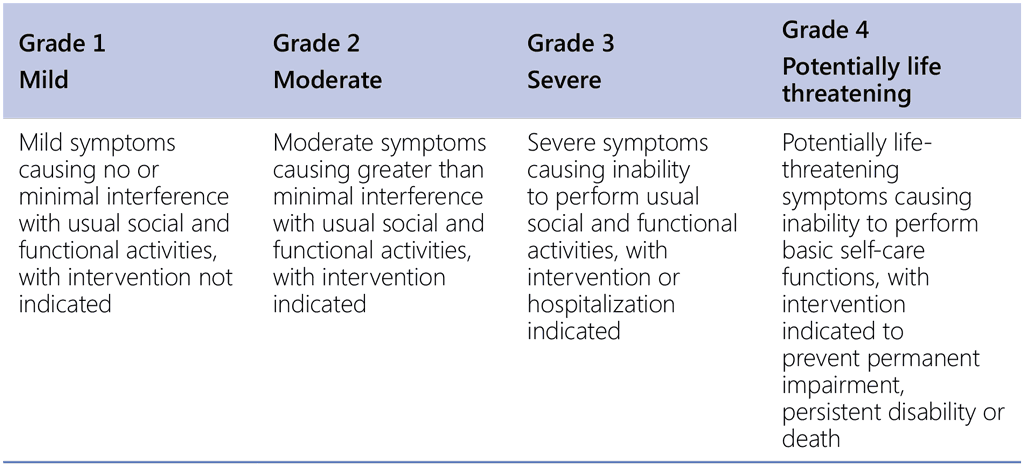
Source: US Department of Health and Human Services, 2017 (27).
Table A2.6. Management of common AEs associated with the BPaLM regimen, according to severity grading
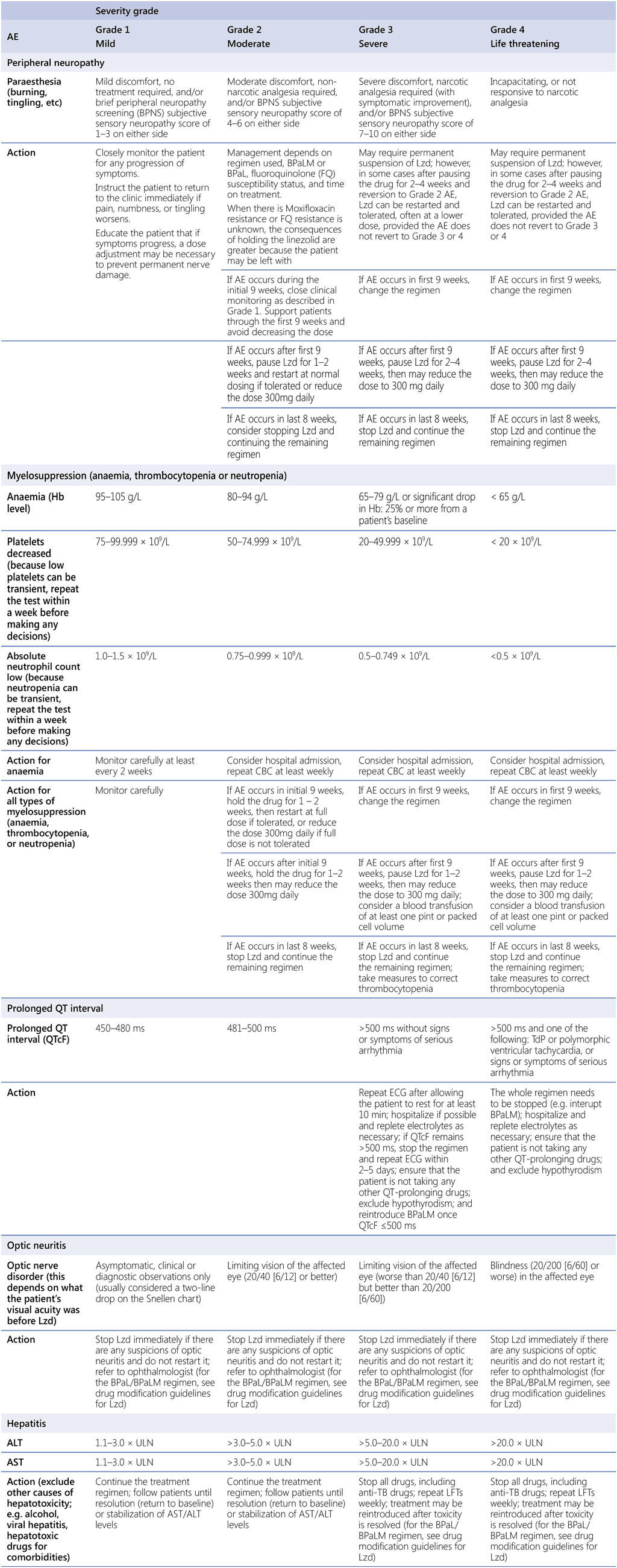
AE: adverse event; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BPaL: bedaquiline, pretomanid and linezolid; BPaLM: bedaquiline, pretomanid, linezolid and moxifloxacin; BPNS: brief peripheral neuropathy screen; CBC: complete blood count; ECG: electrocardiography; Hb: haemoglobin; LFT: liver function test; Lzd: linezolid; MWF: Monday, Wednesday, Friday; QTcF: corrected QT interval per Fridericia’s formula; TB: tuberculosis; TdP: torsades de pointes; ULN: upper limit of normal.
References (Annex 2)
- Active tuberculosis drug-safety monitoring and management (aDSM): framework for implementation. Geneva: World Health Organization; 2015 (https://apps.who.int/iris/bitstream/handle/10665/204465/WHO_HTM_TB_2015.28_eng.pdf).
- Lan Z, Ahmad N, Baghaei P, Barkane L, Benedetti A, Brode SK et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8:383–94. doi: https://doi.org/10.1016/S2213-2600(20)30047-3.
- Borisov S, Danila E, Maryandyshev A, Dalcolmo M, Miliauskas S, Kuksa L et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J. 2019;54:1901522. doi: https://doi.org/10.1183/13993003.01522-2019.
- Tack I, Dumicho A, Ohler L, Shigayeva A, Bulti AB, White K et al. Safety and effectiveness of an all-oral, bedaquiline-based, shorter treatment regimen for rifampicin-resistant tuberculosis in high human immunodeficiency virus (HIV) burden rural South Africa: a retrospective cohort analysis. Clin Infect Dis. 2021;73:e3563–e71. doi: https://doi.org/10.1093/cid/ciaa1894.
- Conradie F, Phillips P, Badat T. High rate of successful outcomes treating RR-TB with a delamanid-bedaquiline regimen in BEAT Tuberculosis: an interim analysis. World Conference on Lung Health 2022. Paris, France: International Union Against Tuberculosis and Lung Disease; 2022.
- Padmapriyadarsini C, Vohra V, Bhatnagar A, Solanki R, Sridhar R, Anande L et al. Bedaquiline, delamanid, linezolid and clofazimine for treatment of pre-extensively drug-resistant tuberculosis. Clin Infect Dis. 2022;76:e938–46. doi: https://doi.org/10.1093/cid/ciac528.
- Schaaf HS, Thee S, van der Laan L, Hesseling AC, Garcia-Prats AJ. Adverse effects of oral second-line antituberculosis drugs in children. Expert Opin Drug Saf. 2016;15:1369–81. doi: https://doi.org/10.1080/1 4740338.2016.1216544.
- Management of drug-resistant tuberculosis in pregnant and peripartum people: a field guide. Boston, USA: The Sentinel Project for Pediatric Drug-Resistant Tuberculosis; 2022.
- Clinical and programmatic guide for patient management with new TB drugs. version 4.0 Paris: endTB Consortium; 2018 (https://endtb.org/sites/default/files/2018-04/Guide%20for%20New%20TB%20Drugs%20Version%204.0.pdf).
- Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142:350–7. doi: https://doi.org/10.3945/jn.111.144287.
- Senneville E, Legout L, Valette M, Yazdanpanah Y, Giraud F, Beltrand E et al. Risk factors for anaemia in patients on prolonged linezolid therapy for chronic osteomyelitis: a case-control study. J Antimicrob Chemother. 2004;54:798–802. doi: https://doi.org/10.1093/jac/dkh409.
- Ishihara S. Ishihara instructions: the series of plates designed as a test for color deficiency. Tokyo: Kanehara & Co., Ltd; No date (https://web.stanford.edu/group/vista/wikiupload/0/0a/Ishihara.14.Plate.Instructions.pdf).
- Azzam D, Ronquillo Y. Snellen chart [website]. Treasure Island (FL): StatPearls Publishing; 2023 (https://www.ncbi.nlm.nih.gov/books/NBK558961/).
- The ASSIST-linked brief intervention for hazardous and harmful substance use: manual for use in primary care. Geneva, World Health Organization. 2012 (https://www.who.int/publications/i/item/the-assist-linked-brief-intervention-for-hazardous-and-harmful-substance-use).
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals. SLACK Incorporated. 2002;32 509–15. doi: https://doi.org/10.3928/0048-5713-20020901-06.
- Tyeku N, Apolisi I, Daniels J, Beko B, Memani B, Cengani L et al. Pediatric delamanid treatment for children with rifampicin-resistant TB. Int J Tuberc Lung Dis. 2022;26:986–8. doi: 10.5588/ijtld.22.0264.
- Summary and recommendations from the second joint meeting of the WHO Global Advisory Committee on Vaccine Safety (GACVS) and Advisory Committee on Safety of Medicinal Products (ACSoMP) 14–16 december 2022. Geneva: 2022 (https://www.who.int/publications/m/item/2022-december-acsomp-recommendations).
- Moore RA, Chi CC, Wiffen PJ, Derry S, Rice ASC. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Db Syst Rev. 2015. doi: https://doi.org/10.1002/14651858.CD010902.pub2.
- Jayabalan B, Low LL. Vitamin B supplementation for diabetic peripheral neuropathy. SMJ. 2016;57:55–9. doi: https://doi.org/10.11622/smedj.2016027.
- Snider DE. Pyridoxine supplementation during isoniazid therapy. Tubercle. 1980;61:191–6. doi: https://doi.org/10.1016/0041-3879(80)90038-0.
- Spellberg B, Yoo T, Bayer AS. Reversal of linezolid-associated cytopenias, but not peripheral neuropathy, by administration of vitamin B6. J Antimicrob Chemother. 2004;54:832–5. doi: https://doi.org/10.1093/jac/dkh405.
- Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893–902. doi: https://doi.org/10.1056/NEJMoa1901814.
- Conradie F, Bagdasaryan TR, Borisov S, Howell P, Mikiashvili L, Ngubane N et al. Bedaquiline–pretomanid– linezolid regimens for drug-resistant tuberculosis. N Engl J Med. 2022;387:810–23. doi: https://doi.org/10.1056/NEJMoa2119430.
- Nyang’wa BT, Berry C, Kazounis E, Motta I, Parpieva N, Tigay Z et al. Short oral regimens for pulmonary rifampicin-resistant tuberculosis (TB-PRACTECAL): an open-label, randomised, controlled, phase 2B-3, multiarm, multicentre, non-inferiority trial. Lancet Respir Med. 2024;12:117–28. doi: https://doi.org/10.1016/S2213-2600(23)00389-2.
- Wares DF, Mbenga M, Mirtskhulava V, Quelapio M, Slyzkyi A, Koppelaar I et al. Introducing BPaL: experiences from countries supported under the LIFT-TB project. PLoS One. 2024;19:e0310773. doi: https://doi.org/10.1371/journal.pone.0310773.
- Lee SS, Lee CM, Kim TH, Kim JJ, Lee JM, Kim HJ et al. Frequency and risk factors of drug-induced liver injury during treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2016;20:800–5. doi: https://doi.org/10.5588/ijtld.15.0668.
- Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events Washington, DC: Division of AIDS National Institute of Allergy and Infectious Diseases. National Institutes of Health. US Department of Health and Human Services; 2017 (https://rsc.niaid.nih.gov/sites/default/files/corrected-grading-table-v-2-1-with-all-changes-highlighted.pdf).
2 Multi-country operational research on the effectiveness and safety of the BPaL regimen for drug-resistant tuberculosis: Unpublished partial cohort analysis (350 individuals enrolled from Indonesia, Kyrgyzstan, the Philippines, Uzbekistan, Vietnam [LIFT-TB initiative], and Nigeria. 2020–2023. KNCV Tuberculosis Foundation (KNCV TB Plus). 2024.
3 Multi-country operational research on the effectiveness and safety of the BPaL regimen for drug-resistant tuberculosis: Unpublished partial cohort analysis (350 individuals enrolled from Indonesia, Kyrgyzstan, the Philippines, Uzbekistan, Vietnam [LIFT-TB initiative], and Nigeria. 2020–2023. KNCV Tuberculosis Foundation (KNCV TB Plus). 2024.
4 The website https://crediblemeds.org/ lists drugs that have a QT-prolonging effect.

 Feedback
Feedback