Book traversal links for 1266
2.4.1 TB preventive treatment
2.4.1.1 Background
People living with HIV have a higher risk of developing TB disease compared to the general population, even when on ART and with high CD4 cell counts. The combined use of TPT and ART has been shown to reduce TB incidence and mortality among people living with HIV, including among those with higher CD4 cell counts (45-47). TPT also provides additional protection when given immediately after the successful completion of treatment for TB disease in people living with HIV (48). TPT should be a core component of the package of care for people living with HIV and should be primarily the responsibility of national HIV and AIDS programmes and HIV service providers (21).
2.4.1.2 Summary of evidence and rationale
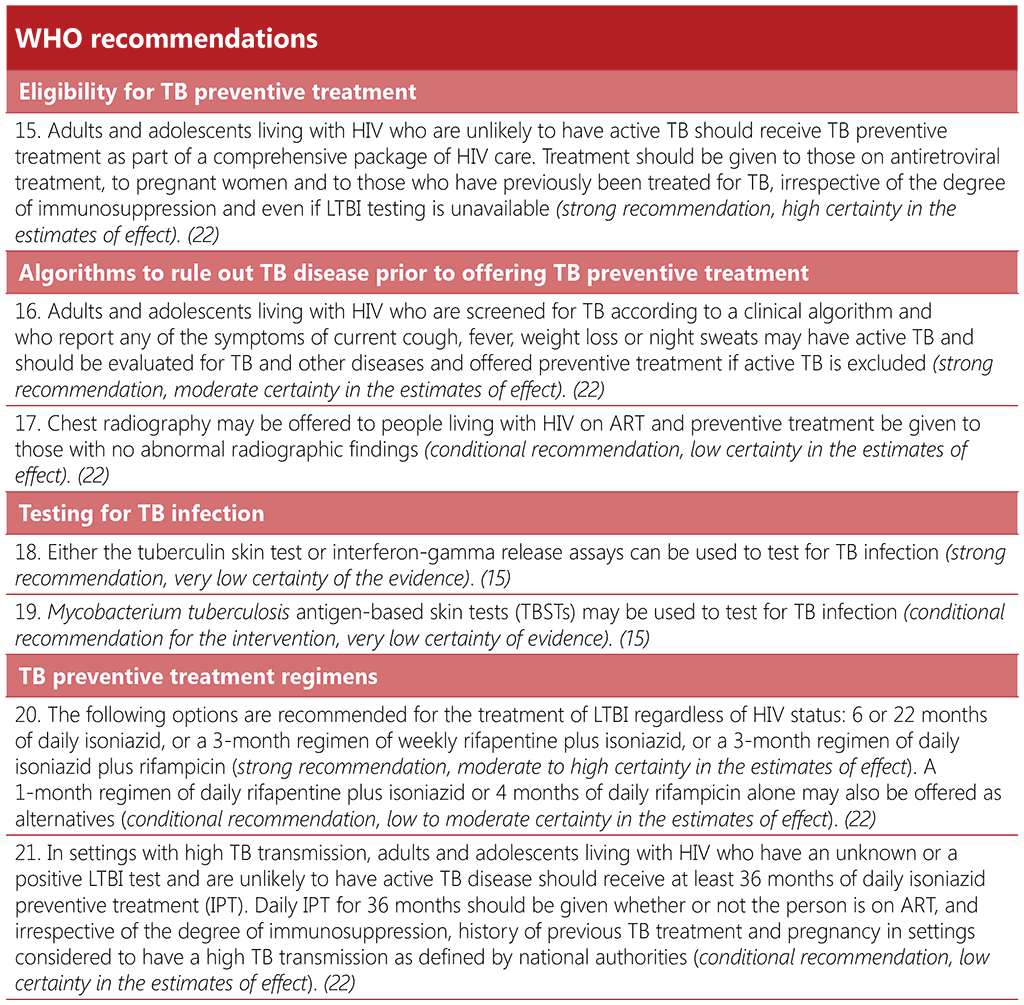
Recommendation 15: TPT for adults and adolescents living with HIV
The recommendation of TPT for all people living with HIV was first published by WHO in 2011 (33). A systematic review of 12 RCTs, which included 8578 people living with HIV, found that preventive treatment reduced the overall risk for TB by 33% (relative risk (RR) 0.67; 95% CI: 0.51–0.87) (49). For those who were TST positive, the reduction increased to 64% (RR 0.36; 95% CI: 0.22–0.61). Although not statistically significant, the reduction was 14% among TST-negative people (RR 0.86; 95% CI: 0.59–1.26) and those of unknown TST status (RR 0.86; 95% CI: 0.48–1.52). Most of the studies in the review were, however, conducted before ART became available, and there is now increasing evidence from observational studies and RCTs of the efficacy of TPT in people receiving ART. TB incidence has been reported to be high among people living with HIV who did not receive IPT, including those with CD4 > 350 cells/mm³ and who were TST negative (50). One double-blind RCT of 1329 people living with HIV receiving ART indicated that those on ART with negative TST or IGRA benefited more from IPT than those who were TST or IGRA positive (45). An RCT of 2056 people living with HIV showed additive benefits of TPT plus ART in reducing both TB incidence and overall mortality (47, 51). The protective effect lasted for more than 5 years.
The GDG reviewed the evidence from the systematic reviews and discussed each population risk group identified in detail for the prevalence of TB infection, risk of progression to TB disease and the incidence of TB disease as compared with the general population. They concluded that the evidence shows a clear benefit of systematic testing and treatment of TB infection for people living with HIV. The wording of the recommendation now refers to TB infection testing rather than TST given that IGRA is also an option (see Recommendation 18), in addition to the recently recommended M. tuberculosis antigen-based skin tests (TBST) (see Recommendation 19). Preventive treatment should be given to adults and adolescents living with HIV, regardless of their immune status and whether they are on ART, given the evidence of additional protective effect when provided with ART. A systematic review of studies conducted before ART became available showed the value of providing preventive treatment immediately after successful completion of TB treatment among people living with HIV in countries with a TB incidence > 100 per 100 000 population (33, 47). Therefore, preventive treatment is recommended for people who were previously treated for TB and for whom a new exposure to TB is confirmed. No evidence was found, however, for preventive treatment of people who had successfully completed treatment for MDR-TB or XDR-TB. The effect of repeated courses of preventive treatment is unclear and hence no recommendation on this is made in the present guidelines. One recent RCT showed that in settings with high TB transmission, a second round of preventive therapy did not provide additional benefit to persons receiving ART (52). In settings with high TB transmission, however, daily IPT for 36 months or longer is recommended conditionally (53) (see Recommendation 21). The relative risk of TB transmission is determined by the local authorities on the basis of risk of exposure (e.g. TB incidence, occurrence of undiagnosed or inadequately treated disease, population density, environmental factors) and host immune response (13).
Pregnant women living with HIV are at risk for TB, which can have severe consequences for both the mother and the fetus, with increased risk of maternal and infant death (54). Pregnancy should not disqualify women living with HIV from receiving preventive treatment with medicines commonly used to treat TB disease that are generally considered safe for use in pregnancy, such as isoniazid and rifampicin (classified as Pregnancy Category C by the United States Food and Drug Administration (FDA)) (55, 56).
Recommendations 16–17: Algorithms to rule out TB disease prior to giving TPT
In 2011, WHO conducted a systematic review and IPD meta-analysis and recommended a symptom-screening rule of a combination of current cough, weight loss, night sweats and fever to exclude TB disease in adults and adolescents (57). The review showed that the rule had a sensitivity of 78.9% (95% CI: 58.3%–90.9%), a specificity of 49.6% (95% CI: 29.2%–70.1%) and a negative predictive value of 97.7% (95% CI: 97.4%–98.0%) at a TB prevalence of 5%. Most people living with HIV in studies included in the systematic review were not receiving ART.
During the 2018 update of the guidelines on TPT, a systematic review was undertaken to compare the performance of the four symptom screen in people living with HIV who were and were not receiving ART (58). Data from 17 studies were included in this analysis. The pooled sensitivity of the four symptom screen for people living with HIV on ART was 51.0% (95% CI: 28.4–73.2), and the specificity was 70.7% (95% CI: 47.7–86.4); in people living with HIV who were not receiving ART the pooled sensitivity was 89.3% (95% CI: 82.6–93.6), and the specificity was 27.2% (95% CI: 17.3–40.0). Two studies provided data on the addition of abnormal chest radiographic findings to the screening rule for people living with HIV on ART (59, 60). The pooled sensitivity was higher (84.6%, 95% CI: 69.7–92.9), but the specificity was lower (29.8%, 95% CI: 26.3–33.6) when compared with the symptom screen alone.
In all studies, the median prevalence of TB among people living with HIV on ART was 1.5% (interquartile range: 0.6–3.5%). At a 1% prevalence of TB, the negative predictive value of the symptom screening rule was 99.3%; addition of abnormal chest radiographic findings increased the negative predictive value by 0.2%. No studies of the addition of chest radiography to the symptom rule for pregnant women were found in the review.
During the development of the 2020 updated guidelines, the GDG agreed that in adults and adolescents living with HIV the four symptom screen – current cough, fever, weight loss or night sweats – is very useful for ruling out TB disease, regardless of ART use. Confirmation of TB infection would be desirable before starting TPT, although lack of access to TB infection testing should not be a barrier to TPT initiation. It noted the potential benefits of adding an abnormal chest radiographic finding to the rule, while recognizing that the improvement in performance was marginal. Moreover, increased use of chest radiography would add more false-positive results to the screening rule, which would require more investigations for TB and other illnesses. Therefore, the GDG reiterated that chest radiography may be added as an additional investigation only if it does not pose a barrier to the provision of preventive treatment for people living with HIV. It should not be a requirement for initiating preventive treatment. Although no study was found of the additive role of chest radiography in testing pregnant women, the GDG noted that pregnant women living with HIV could also benefit, as long as good practices are observed to prevent harmful radiation exposure to the fetus (61).
Recommendation 18: IGRA and TST for testing for TB infection
In 2011, WHO issued recommendations on the use of IGRAs for the diagnosis of TB infection, including the blood-based QIAGEN QuantiFERON®-TB Gold (QFT-G), QIAGEN QuantiFERON-TB Gold In-Tube (QFT-GIT) and Oxford Immunotec T-SPOT®.TB (T-Spot) (62) assays. In 2018, WHO updated the recommendations to stipulate that the TST or IGRAs (or both) can be used to test for TB infection in LMICs. The recommendation on IGRA for use as a test for infection was first published in the 2018 WHO guidelines (63). A previous systematic review was updated to compare the predictive performance of IGRA and TST for identifying incident TB disease in countries with a TB incidence > 100 per 100 000 population (64). Only studies in which TST was compared with IGRA in the same population (“head-to-head” studies) were included. Relative risk ratios for TB for people who tested positive and those who tested negative with TST and IGRA were estimated.
Five prospective cohort studies were identified, with a total of 7769 participants; four were newly identified. Three of the studies were conducted in South Africa and two in India (45, 65-68). The studies included people living with HIV, pregnant women, adolescents, healthcare workers and household contacts. The pooled risk ratio estimate for TST was 1.49 (95% CI: 0.79–2.80), and that for IGRA was 2.03 (95% CI: 1.18–3.50). Although the estimate for IGRA was slightly higher than that for TST, the 95% CIs for the estimates for TST and IGRA overlapped and were imprecise. Furthermore, there was limited evidence for the predictive utility of the tests in specific at-risk populations.
The evidence reviewed and the recommendations apply only to the use of the two commercially available IGRAs (QuantiFERON®-TB Gold In-Tube and T-SPOT®.TB). The GDG concluded that the comparison of TST and IGRA in the same population does not provide strong evidence that one test should be preferred over the other for predicting progression to TB disease. TST may require significantly fewer resources than IGRA and may be more familiar to practitioners in resource-constrained settings; however, recurrent global shortages and stock-outs of TST reduce prospects for its scale-up in programmatic management of TPT.
The GDG cautioned that imperfect performance of these tests can lead to false-negative results, particularly for young children and immunocompromised individuals such as people living with HIV with low CD4 counts. Although some studies suggest otherwise (45, 50), the GDG maintained the past position that people living with HIV who have a positive test for TB infection benefit more from TPT than those who have a negative TB infection test (33, 63). TB infection testing can be used, where feasible, to identify such individuals. However, based upon evidence of moderate certainty, the GDG strongly emphasized that TB infection testing by TST or IGRA should not be a prerequisite to start TPT in people living with HIV and household contacts aged < 5 years, particularly in settings with a high TB incidence (e.g. > 100 TB cases per 100 000 population), given that benefits clearly outweigh the risks. A negative TB infection test in these two groups, as well as in HIV-negative infant household contacts, should be followed by a case-by-case assessment for the potential benefit and harms of TPT.
Recommendation 19: Mycobacterium tuberculosis antigen-based skin tests for testing for TB infection
In 2022, WHO issued recommendations on the use of TBST for the diagnosis of TB infection. To inform these recommendations WHO commissioned a systematic review in 2021 of published and unpublished data on this new class of tests for TB infection not previously reviewed by WHO. The technologies that were included in the evaluation were Cy-Tb (Serum Institute of India, India), Diaskintest® (Generium, Russian Federation) and C-TST (formerly known as ESAT6-CFP10 test, Anhui Zhifei Longcom, China). This more recent class of TB infection tests is defined as in vivo skin tests for the detection of TB infection that use M. tuberculosis-specific antigens (ESAT-6 and CFP-10).
Based on available evidence, in 2022 the WHO GDG panel concluded that the diagnostic accuracy of TBSTs is similar to that of IGRAs and greater than that of the TST. The GDG panel expressed concerns about the certainty (quality) of evidence in many areas and the lack of longitudinal studies that include impact on people affected by important outcomes of TB. The risk of bias was primarily from non-blinded studies, and the quantity and quality of evidence varied among the different tests. For two of the three tests evaluated during the GDG meeting (Diaskintest® and C-TST), evidence on specificity was generated in high TB burden settings; therefore, additional analysis considered the concordance in specificity with existing WHO-recommended IGRAs. All three evaluated TBSTs have the potential to be used for the detection of TB infection and are recommended. No safety concerns were identified for the class of tests; however, evaluation and approval by the competent regulatory agencies for the individual products are essential before introduction of these in vivo tests. Although the data were limited, based on the available evidence, the GDG members supported extrapolation of the recommendation for people living with HIV. Further details, including on safety, cost analysis and user perspective, can be found in the WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – tests for tuberculosis infection (15).
Recommendation 20: TPT regimens
Daily isoniazid monotherapy
The efficacy of six months daily isoniazid monotherapy (6H) in different populations and settings has been shown in a number of systematic reviews (49, 69, 70). A systematic review of RCTs in people living with HIV showed isoniazid monotherapy reduces the overall risk for TB by 33% (RR 0.67; 95% CI: 0.51–0.87), and that the preventive efficacy reached 64% for people with a positive TST (RR 0.36; 95% CI: 0.22–0.61) (49). Furthermore, the efficacy of the 6-month regimen was not significantly different from that of 12 months’ daily isoniazid monotherapy (RR 0.58; 95% CI: 0.3–1.12). A systematic review of RCTs also showed a significantly greater reduction in TB incidence among participants given the 6-month regimen than in those given a placebo (odds ratio (OR) 0.65; 95% CI: 0.50–0.83) (71). No controlled clinical trials were found of daily isoniazid monotherapy for 9 months (9H) versus 6H. Re-analysis and modelling of the United States Public Health Service trials of isoniazid conducted in the 1950s and 1960s, however, showed that the benefit of isoniazid increases progressively when it is given for up to 9–10 months and stabilizes thereafter (72). For this reason, 9H is retained as an alternative regimen to 6H in the recommended TPT options.
Daily rifampicin plus isoniazid for 3 months (3HR)
A systematic review updated in 2017 showed that the efficacy and the safety profile of 3–4 months’ daily rifampicin plus isoniazid were similar to those of 6 months’ isoniazid (71, 73). A previous GDG therefore strongly recommended that daily rifampicin plus isoniazid could be used as an alternative to isoniazid in settings with a TB incidence < 100 per 100 000 population (74).
Daily rifampicin monotherapy for 4 months (4R)
A previous systematic review conducted for the 2015 guidelines on TPT and updated in 2017, found similar efficacy for 3–4 months’ daily rifampicin and 6H (OR 0.78; 95% CI: 0.41–1.46) (71, 73). The review also showed that individuals given rifampicin daily for 3–4 months had a lower risk for hepatotoxicity than those treated with isoniazid monotherapy (OR 0.03; 95% CI: 0.00–0.48).
In 2019, the GDG discussed the implications of using 4R in high TB burden settings based on findings from RCTs of 4R vs 9H that included adults and children from such countries (75-78). In study participants > 17 years, the difference in rate of confirmed TB between 4R and 9H (4R arm minus 9H arm) was < 0.01 cases per 100 person-years (95% CI: −0.14 to –0.16); the difference in treatment completion was 15.1% (95% CI: 12.7–17.4); the difference for Grade 3–5 adverse events was −1.1% (95% CI: −1.9 to –0.4). In individuals < 18 years, the difference in rate of TB disease between 4R and 9H was –0.37 cases per 100 person-years (95% CI: −0.88 to 0.14); the difference in treatment completion was 13.4% (95% CI: 7.5–19.3); the difference in risk for adverse events attributed to the medicine used and resulting in discontinuation was −0.0 (95% CI: −0.1 to 0.1).
Daily rifapentine plus isoniazid for 1 month (1HP)
In 2019, the GDG considered data from the only known published study of the 1HP regimen: a randomized, open-label, phase 3 non-inferiority trial comparing the efficacy and safety of 1HP with 9 months of isoniazid alone (9H) in people living with HIV in high TB prevalence settings or who had evidence of TB infection (78). Enrolment was restricted to individuals ≥ 13 years old who were not pregnant or breastfeeding. Non-inferiority would be shown if the upper limit of the 95% CI for the between-group difference in the number of events per 100 person-years was less than 1.25.
Among all study participants, the difference in incidence rate of TB (including deaths from any cause) between 1HP and 9H (1HP arm minus 9H arm) was −0.02 per 100 person-years (95% CI: −0.35 to 0.30); the relative risk (RR) for treatment completion of 1HP over 9H was 1.04 (95% CI: 0.99–1.10); the RR for Grade 3–5 adverse events was 0.86 (95% CI: 0.58–1.27); hazard ratio of death from any cause was 0.75 in favour of 1HP (95% CI: 0.42–1.31); RR for emergence of resistance to isoniazid and rifampicin were, respectively, 1.63 (95% CI: 0.17–15.99) and 0.81 (95% CI: 0.06–11.77). Overall non-inferiority as defined by the study protocol was thus shown in the modified intention to treat (mITT) population. Non-inferiority was also shown for the sub-group with confirmed TB infection (incidence rate difference per 100 person-years was 0.069 (–0.830 to 0.690)), as well as in males and females, and among those on or without ART at start of study. The number of patients with CD4 < 250 cells/ mm3 was small, and neither inferiority nor non-inferiority of 1HP was shown in this stratum.
Weekly rifapentine plus isoniazid for 3 months (3HP)
A systematic review was conducted for the 2018 guidelines update to compare the effectiveness of a 3-month weekly regimen of rifapentine plus isoniazid (3HP) with that of isoniazid monotherapy. The review covered four RCTs (79-82), which were analyzed for three subgroups, including adults living with HIV.
Two of the RCTs involved adults with HIV from South Africa, Peru and a number of countries with a TB incidence < 100 per 100 000 population. No significant difference was found in the incidence of TB disease between participants given a 3HP and 6H or 9H (RR 0.73; 95% CI: 0.23–2.30). Furthermore, the risk for hepatotoxicity was significantly lower with 3HP in adults living with HIV (RR 0.26; 95% CI: 0.12–0.55). The 3HP regimen was also associated with a higher completion rate in all subgroups (adults with HIV: RR 1.25; 95% CI: 1.01–1.55). One RCT included a comparison between 3HP and continuous isoniazid monotherapy in adult people living with HIV (79). No significant difference in TB incidence was found in an intention-to-treat analysis; however, a per-protocol analysis showed a lower rate of TB infection or death in participants given continuous isoniazid. In all the studies, 3HP was given under direct observation.
Recommendation 21: 36 months of daily isoniazid monotherapy
A systematic review and meta-analysis of three RCTs of people living with HIV in settings with high TB prevalence and transmission showed that continuous IPT can reduce the risk for TB disease by 38% more than 6 months’ isoniazid (83). The effect was greater in people with a positive TST (49% for TB disease and 50% for death). In those with a negative TST, neither effect was significant, although the point estimate indicated a reduction in TB incidence of 27%. In two of the studies reviewed, ART was not used and in the third ART coverage was low at baseline but increased during the period of observation.
This recommendation is conditional and based on evidence that longer-term IPT significantly adds benefit to ART. The efficacy, safety and convenience of repeated treatment with shorter rifapentine regimens is being studied in people living with HIV in such settings. The definition of a high TB transmission setting should be established by the national authorities. Testing for TB infection is not a prerequisite for TPT in people living with HIV but its use is encouraged because people who are TST positive have a greater protective benefit from TPT. People living with HIV with a negative TST should not receive 36 months of daily IPT.
Special considerations
Careful consideration should be given to the selection of TPT regimen for people living with HIV. Rifamycins induce certain cytochrome P-450 enzymes and may therefore accelerate the elimination of medicines that depend on this metabolic pathway, including several antiretroviral drugs (ARVs) (22).
These regimens should not be administered to people receiving protease inhibitors or nevirapine, including for HIV-exposed infants on TPT. Rifampicin can decrease the concentrations of other antiviral agents: atazanavir, darunavir, fosamprenavir, lopinavir, saquinavir and tipranavir. It should not be used with saquinavir/ritonavir. No dose adjustment is required when rifampicin is co-administered with efavirenz. The dose of dolutegravir (DTG) however needs to be increased to 50 mg twice daily when given together with rifampicin, a dose that is usually well tolerated and gives equivalent efficacy in viral suppression and recovery of CD4 cell count compared with efavirenz (22).
The 3HP regimen can be administered to individuals receiving efavirenz-based antiretroviral regimens without dose adjustment, according to a study of pharmacokinetics (84). Administration of rifapentine with raltegravir was found to be safe and well tolerated (85). A drug interaction study in healthy volunteers of DTG with once weekly HP reported toxicities in two of four participants (86). However results released more recently from a Phase 1/2 trial of 3HP and DTG in adults with HIV reported good tolerance and viral load suppression, no adverse events of Grade > 3 related to 3HP, and did not indicate that rifapentine reduced DTG levels sufficiently to require dose adjustment (87).
Pregnant women living with HIV are at risk for TB, which can have severe consequences for both the mother and the fetus, with increased risk of maternal and infant death (54). Pregnancy should not disqualify women living with HIV from receiving preventive treatment with medicines commonly used to treat TB that are generally considered safe for use in pregnancy, such as isoniazid and rifampicin (classified as Pregnancy Category C by the United States FDA (55, 56). There are limited data on the efficacy and safety of rifapentine during pregnancy. WHO currently recommends six months of isoniazid regimen as TPT for pregnant women living with HIV. A systematic review in 2019 identified one RCT and three non-randomized comparative observational studies that provided data on adverse pregnancy outcomes associated with the use of IPT among pregnant women living with HIV. While the RCT showed a higher risk of adverse pregnancy outcomes among those who initiated IPT during pregnancy (Mantel-Haenszel OR stratified by gestational age, 1.51; 95% CI: 1.09–2.10), all three other studies reported an overall OR < 1 suggesting the opposite (I2=80%, p=0.002). A meta-analysis from two observational studies that cited adjusted estimates and whose data could be pooled suggested lower risk for composite adverse pregnancy outcomes (OR 0.40; 95% CI: 0.20–0.74) (88). Based upon these findings the GDG concluded that there were insufficient grounds to change previous guidance or to develop a separate recommendation for the use of IPT in pregnant women living with HIV. The GDG considered that systematic deferral of IPT to the postpartum period would deprive people from its protective effect at a point when they are more vulnerable to TB.
Concurrent use of alcohol should be avoided with TPT.
Further details on drug-drug interactions for TPT and TB treatment regimens are provided in the WHO operational handbook on tuberculosis. Module 6: tuberculosis and comorbidities (7).
2.4.2 TB infection prevention and control
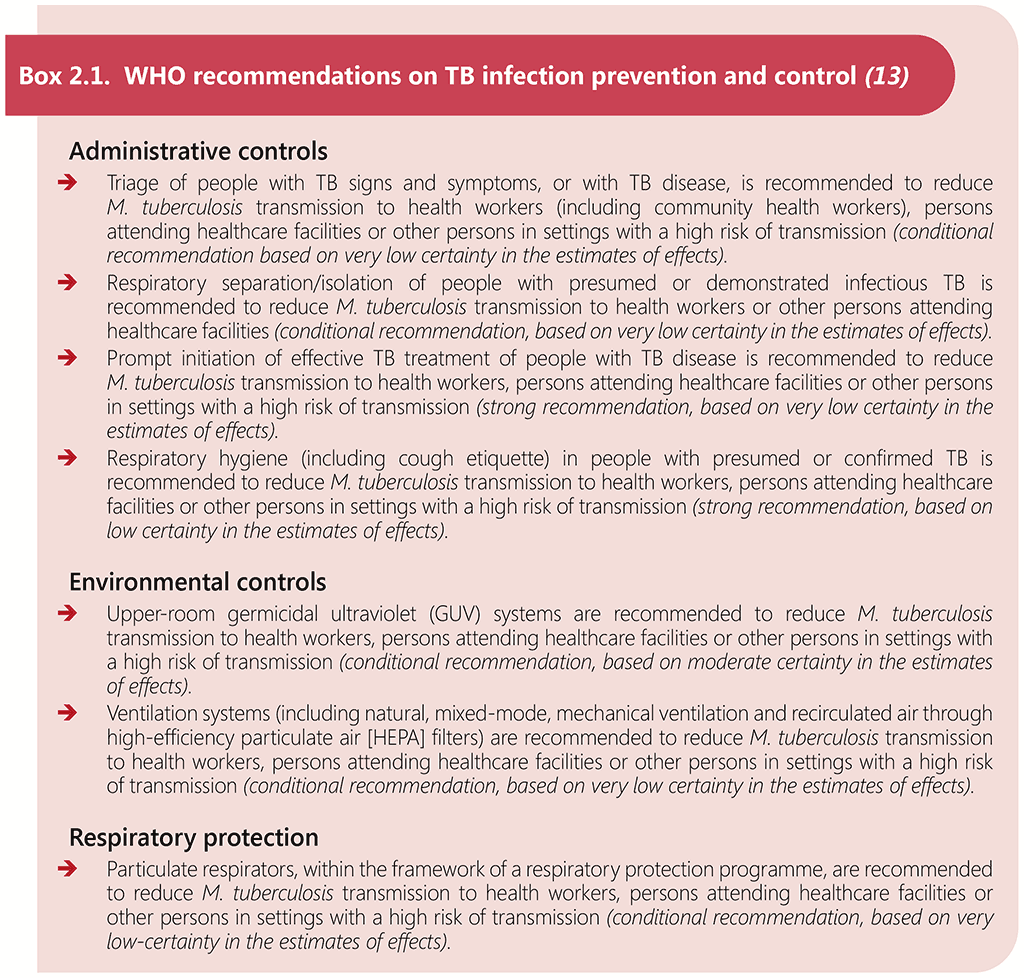
Comprehensive infection prevention and control (IPC) measures are essential to prevent TB transmission in clinical settings that provide services for people living with HIV (13). Whilst there are no recommendations specifically for TB IPC in HIV care settings, the general TB IPC recommendations are relevant and are listed in Box 2.1 below. Also of relevance are the WHO Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level (89), which cover IPC measures preventing transmission of infectious diseases that apply to all healthcare settings.
2.4.2.1 Summary of evidence and rationale
Healthcare facilities and congregate settings can present a high risk for acquiring TB (including MDR-TB) for people living with HIV as well as for healthcare workers. Evidence has shown an increased risk of TB due to HIV among healthcare workers as well as medical and nursing students with patient contact (90). Further, studies have highlighted the role of HIV in fuelling the TB epidemic among people in prison (91) and refugees and internally displaced people living in crowded camps or detention centres (92).
The WHO consolidated guidelines on tuberculosis. Module 1: prevention – infection prevention and control (13) comprises (i) a set of core components of IPC programmes, and (ii) a set of TB-specific interventions to reduce transmission of M. tuberculosis at the facility level. The core components include recommendations that should underpin all activities aimed at reducing healthcare-associated infections and antimicrobial resistance, including for TB, while the TB-specific interventions comprise recommendations on administrative controls, environmental controls and respiratory protection measures to reduce TB transmission in high-risk setting (13). Administrative controls aim to reduce the risk of exposure to persons with infectious TB; recommended interventions include triage of people with signs and symptoms of TB, respiratory isolation of people with presumed or demonstrated infectious TB, prompt initiation of effective treatment, and education on respiratory hygiene including cough etiquette. Environmental controls aim to prevent the spread of infectious respiratory particles and reduce their concentration; recommended interventions include the use of upper-room germicidal ultraviolet (GUV) systems and maximizing ventilation. Respiratory protection measures comprise the use of personal protective equipment, including particulate respirators, in situations that pose a high risk of exposure to M. tuberculosis.
The WHO consolidated guidelines on tuberculosis. Module 1: prevention – infection prevention and control (13) provides recommendations and supporting evidence on preventing the transmission of TB in healthcare and other congregate settings, through administrative controls, environmental controls and respiratory protection measures, and the WHO operational handbook on tuberculosis. Module 1: prevention – infection prevention and control (93) provides implementation guidance. The WHO Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level (89) provides additional detail on IPC measures preventing transmission of infectious diseases that apply to all healthcare settings.

 Feedback
Feedback