Book traversal links for WHO TB KNOWLEDGE SHARING PLATFORM
2.3.1 Background
Early initiation of TB treatment and ART among people with both TB and HIV is critical for reducing mortality and improving TB treatment outcomes. People living with HIV who receive a diagnosis of TB should receive a WHO-recommended TB treatment regimen. This section covers timing of TB treatment, as well as provision of integrated care. WHO recommendations on treatment regimens for people with drug-susceptible TB and MDR-TB can be found in WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-susceptible tuberculosis treatment (17) and in WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment (16). The timing of ART initiation in people with presumed or diagnosed TB is covered in Section 3.2.
2.3.2 Summary of evidence and rationale
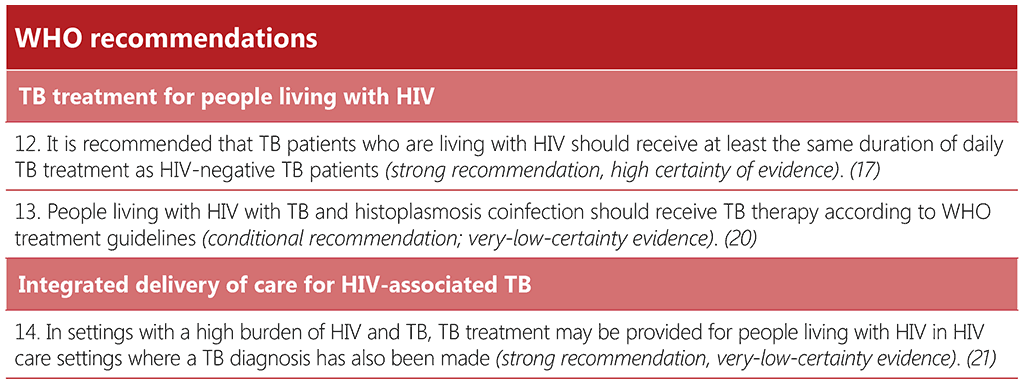
Recommendation 12: Duration of daily TB treatment for people living with HIV
This recommendation was first put forward in 2010 and is considered valid in the guidelines update of 2017 and in the current consolidated guidelines on drug susceptible TB treatment. A systematic review and meta-analysis of six randomized controlled trials (RCTs) and 21 cohort studies provided pooled estimates of failure, relapse and death by duration of rifampicin, and daily intensive phase versus intermittent throughout (40). The systematic review revealed a marked and significant reduction in failure and relapse in the study arms in which some or all study participants received ART. In a regression model, treatment failure or relapse was 1.8–2.5 times more likely with intermittent rather than daily dosing in the intensive phase. Compared with 8 or more months of rifampicin, 2-month rifampicin regimens carried a 3-fold higher risk of relapse, and 6-month regimens carried a 2.2-fold higher risk. Extending treatment beyond 6 months is recommended by some expert groups in certain persons living with HIV and the meta-analysis showed that this is associated with significantly lower relapse rates. However, several other considerations were given greater weight. Separate regimens for people with TB living with or without HIV would be very challenging in operational terms and could create stigma. Other potential harms of extending treatment are acquired resistance to rifampicin, and a longer period during which ART options are limited (because of ART–rifampicin interactions).
Recommendation 13: TB treatment for people living with HIV and histoplasmosis
Histoplasmosis is highly endemic in some parts of the WHO Region of the Americas and is also reported in certain countries of Asia and Africa (20). Co-occurrence can lead to complex management, with drug-drug interactions that may affect HIV, TB, and histoplasmosis treatment (41). In particular, rifampicin results in reduced itraconazole levels, potentially leading to ineffective treatment for histoplasmosis (42).
A systematic review that informed the development of the Pan American Health Organization (PAHO) and WHO Guidelines for diagnosing and managing disseminated histoplasmosis among people living with HIV (20) found two studies (including one case report) reporting on treatment outcomes among people living with HIV, histoplasmosis and TB (42, 43). This recommendation therefore relies on the expertise of the GDG and considers existing guidance on managing HIV and TB disease. The recommendation balances the risk for acquisition of TB drug resistance and the risk of drug-drug interactions (rifampicin and itraconazole), leading to subtherapeutic itraconazole levels and potential ineffective treatment for histoplasmosis.
When histoplasmosis is not controlled because of interactions between rifampicin and itraconazole, clinicians may consider, depending on local context, extending the duration of amphotericin B induction therapy, once-weekly courses of amphotericin B, increasing the itraconazole dose and monitoring the blood level and toxicity and considering using other azole drugs (osaconazole, voriconazole, or fluconazole). Finally, clinicians can consider replacing rifampicin with rifabutin. Treatment may need to be revised for people experiencing toxicity, drug-drug interactions, or for those with resistance profiles requiring protease inhibitors or second-line TB drugs. When possible, antiretroviral resistance genotyping and TB drug susceptibility testing may assist clinical decisions. Itraconazole serum level testing may not be available in some areas.
Recommendation 14: Providing TB treatment in HIV care settings
A systematic review evaluating the effectiveness of delivering ART in TB treatment settings identified 19 observational studies, many of which showed increased uptake and timeliness of ART initiation. However, the data on mortality and TB treatment success were inconsistent. The same systematic review identified five observational studies evaluating the effectiveness of delivering TB treatment in HIV care settings. Two studies reported decreased mortality and another showed comparable mortality rates. The TB treatment success rates and ART uptake were comparable across studies (44).

 Feedback
Feedback