Book traversal links for WHO TB KNOWLEDGE SHARING PLATFORM
2.2.1 Background
People living with HIV may have an atypical clinical picture, especially those with advanced HIV disease, complicating the diagnosis of pulmonary and extrapulmonary forms of TB disease. Access to a rapid and accurate diagnosis is essential to ensure that TB is effectively treated among people living with HIV.
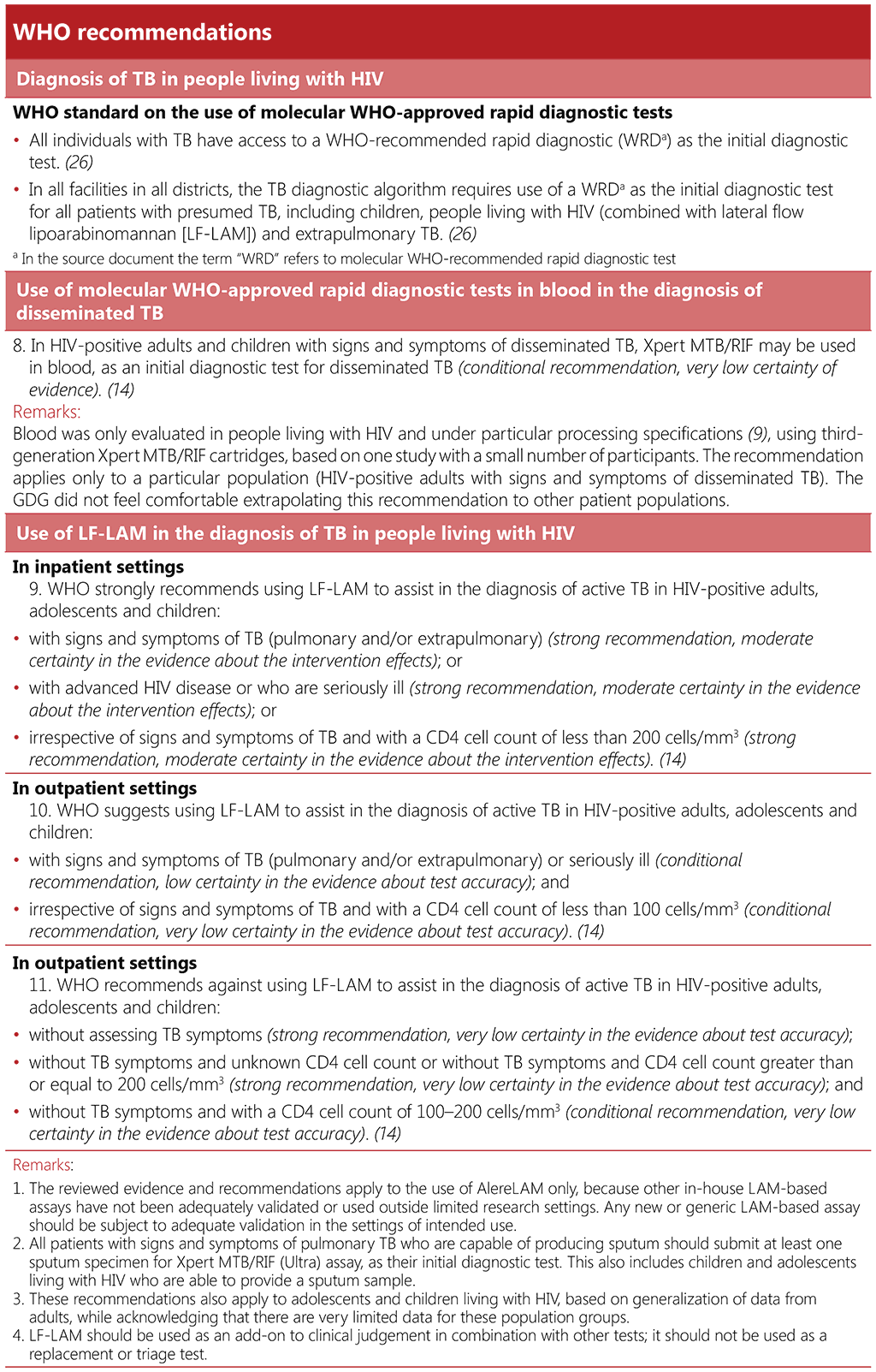
Options for TB diagnosis recommended by WHO comprise two broad groups: i) initial tests for diagnosing TB, often including at least rifampicin resistance detection, and ii) follow-on tests used after TB confirmation to detect additional drug resistance. These guidelines focus on the first category. Table 2.6 summarizes the initial WHO-recommended rapid diagnostic tests for TB, which are applicable for everyone, except LF-LAM and the use of mWRD in blood which are specific to people living with HIV. Further details on the accuracy of all the tests as well as the follow-on tests to detect additional drug resistance can be found in WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection, 2021 update (14).
Table 2.6 WHO-recommended rapid diagnostic tests as initial tests for TB diagnosis
In many high TB burden settings, sputum-smear microscopy remains the primary diagnostic tool for evaluating individuals presenting with signs and symptoms of TB. However, sputum-smear microscopy has a low sensitivity up to approximately 50% among people living with HIV (34, 35), who often have difficulty in producing sputum or have paucibacillary sputum. The sensitivity will vary with the setting and as well as with the degree of immunosuppression of the individual. Furthermore, sputum-smear microscopy cannot distinguish drug-susceptible strains from drug-resistant strains. WHO recommends that TB programmes transition to replacing microscopy as the initial diagnostic test with mWRDs that detect Mycobacterium tuberculosis (M. tuberculosis) complex bacteria (MTBC). The WHO standard: universal access to rapid tuberculosis diagnostics includes two benchmarks relating to access to mWRDs as an initial diagnostic test, including one that requires the use of mWRD as an initial diagnostic test, combined with urinary LF-LAM, for people living with HIV (26).
2.2.2 Summary of evidence and rationale
Recommendation 8: use of molecular WHO-recommended rapid diagnostic tests
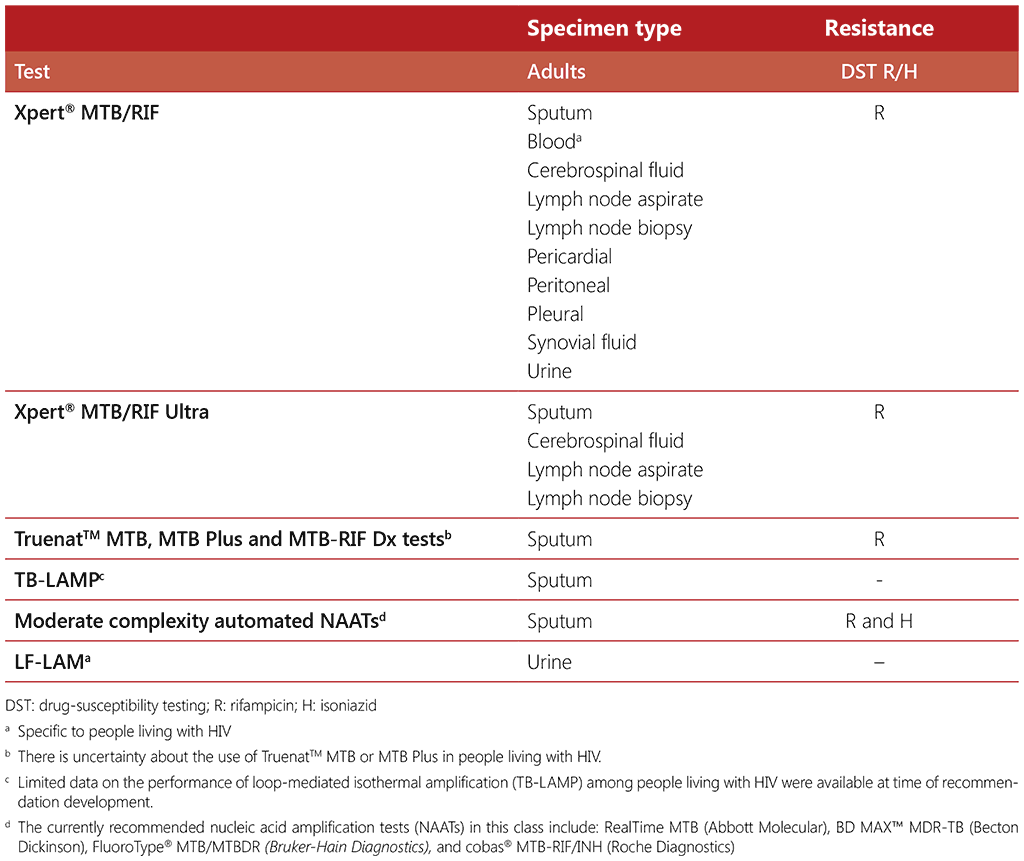
mWRDs incorporate a growing number of different products that detect M. tuberculosis genetic material in samples. Most mWRDs detect rifampicin resistance, while some also detect isoniazid resistance. Table 2.7 summarizes the evidence on the accuracy of the different tests in the diagnosis of TB in people living with HIV.
Table 2.7 Diagnostic accuracy of mWRDs for TB diagnosis in people living with HIV compared with microbiological reference standard
In 2011 WHO first recommended the use of Xpert MTB/RIF, as the initial diagnostic test using sputum for pulmonary TB in individuals suspected of MDR-TB or HIV-associated TB (36). This strong recommendation was updated in 2013, based on high-quality evidence and increased accuracy, recommending that Xpert MTB/RIF should be used rather than conventional microscopy, culture and drug-susceptibility testing, as the initial diagnostic test in adults suspected of having MDR-TB or HIV-associated TB (36). Since 2020, WHO has recommended a number of mWRDs for the initial diagnosis of TB, instead of smear microscopy, for all people being evaluated for pulmonary and extrapulmonary TB, regardless of HIV status (37).
WHO recommends that mWRDs can be used for testing the following non-respiratory specimens for people presenting with signs and symptoms of extrapulmonary TB: cerebrospinal fluid (strong recommendation), lymph node samples, pleural, peritoneal, pericardial, synovial fluid or urine (conditional recommendations). Of the total 65 studies that reviewed data on the diagnosis of extrapulmonary TB, 41 studies (63%) took place in high burden TB/HIV countries. Although data in the evaluation are not disaggregated by HIV status these recommendations also apply to people living with HIV.
The use of mWRD to test blood is recommended specifically for people living with HIV who present with signs and symptoms of disseminated TB. For the 2021 update of the WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection, 2021 update (14), the use of mWRD in blood was only evaluated in people living with HIV and under particular processing specifications using third-generation Xpert MTB/RIF cartridges, based on one study with a small number of participants (38).
At the time of the guideline development for recommendations on loop-mediated isothermal amplification (TB-LAMP), the assay was found to have limited additional diagnostic value over sputum-smear microscopy for testing people living with HIV, however a further review is planned. It was also emphasized that TB-LAMP should not replace the use of rapid molecular tests that have a higher sensitivity for the detection of TB among people living with HIV who have signs and symptoms consistent with TB.
There is some uncertainty about the use of TruenatTM (MTB, MTBPlus and MTB-RIF) in people living with HIV, given that there were no HIV-specific data on accuracy of the version of TruenatTM that was assessed during the guideline development. The recommendation on the use of TruenatTM (MTB, MTBPlus and MTB-RIF) in people living with HIV is thus based on extrapolation of the data on test performance with smear-negative sputum specimens.
Recommendations 9–11: Lateral flow urine lipoarabinomannan assay
LF-LAM is a point-of-care test to assist in the diagnosis of TB, specifically used among people living with HIV. It is performed on a urine sample, based on the detection of the lipoarabinomannan (LAM) antigen, and is suitable for use as part of the standard package of care for people with advanced HIV disease. At the time of writing, the Alere Determine TB LAM Ag (AlereLAM) is the only commercially available urine LF-LAM test endorsed by WHO. Details on the use of LF-LAM are provided in the TB diagnostic guidelines (14) and accompanying handbook (39).
As part of a WHO process to update guidelines for the use of the AlereLAM assay, WHO commissioned a systematic review to summarize the current scientific literature on the accuracy of AlereLAM for the diagnosis of TB in people living with HIV. The review identified 15 unique published studies that assessed the accuracy of AlereLAM in adults and integrated nine new studies identified since the original WHO and Cochrane reviews in 2015 and 2016, respectively (10, 11). All studies were performed in high TB/HIV burden countries that were classified as low-income or middle-income countries.
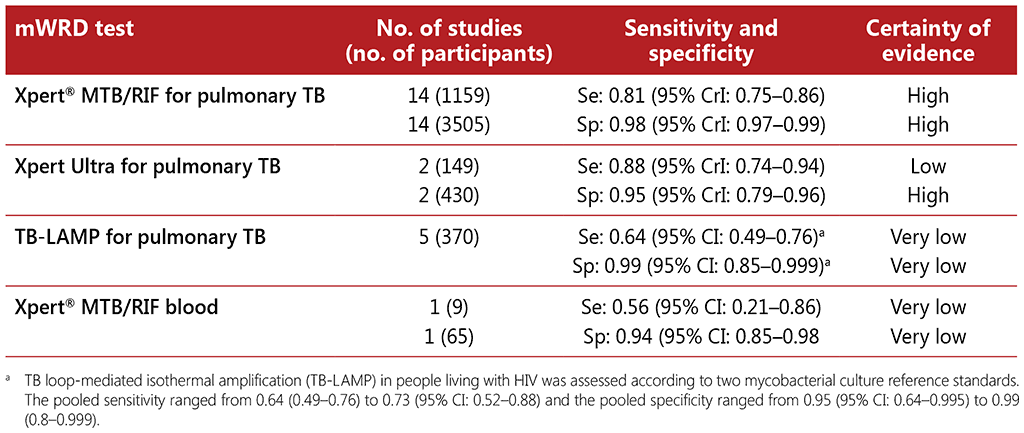
The 15 included studies involved 6814 participants, of whom 1761 (26%) had TB. Eight of the studies evaluated the accuracy of AlereLAM for TB diagnosis in participants with signs and symptoms suggestive of TB; these studies involved 3449 participants, of whom 1277 (37%) had TB. Seven studies evaluated the accuracy of AlereLAM for diagnosis of unselected participants who may or may not have had TB signs and symptoms at enrolment; these studies involved 3365 participants, of whom 439 (13%) had TB. Table 2.8 presents pooled sensitivity and specificity results for AlereLAM against a microbiological reference standard grouped by the study population, TB diagnosis among “symptomatic participants” and TB diagnosis among “unselected participants”.
Unlike traditional diagnostic methods, evidence demonstrates improved sensitivity in people living with HIV with low CD4 cell counts. In addition, the pooled risk ratio from two randomized trials on the impact of AlereLAM in reducing mortality associated with advanced HIV disease was 0.85 (0.76– 0.94); and the absolute effect was 35 fewer deaths per 1000 (from 14 fewer to 55 fewer). Economic evidence for the implementation and scale-up of LF-LAM is limited. The studies that have been done show a consistent trend, suggesting that LF-LAM could be cost-effective in a population of African adults living with HIV (particularly among hospitalized patients). More details are given in web annex 4.13 of the WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics tuberculosis detection: “Economic evaluations of LF-LAM for the diagnosis of active tuberculosis in HIV-positive individuals: an updated systematic review”.
Table 2.8 Diagnostic accuracy of urine LF-LAM for diagnosis of TB among different subpopulations of people living with HIV compared to culture as a reference standard (14)

 Feedback
Feedback