Book traversal links for WHO TB KNOWLEDGE SHARING PLATFORM
2.2.1 Low-complexity automated NAATs
The class of LC-aNAATs is defined in Table 2.3. The number of tests meeting the criteria for this class is expected to grow over the coming years; this will encourage a competitive environment for better product maintenance and support, more options for varying settings and lower prices. New products that meet the class definition according to a WHO/GTB rapid assessment are referred for WHO PQ (17). The list of products currently under evaluation can be reviewed on the WHO PQ website. Products that are successfully WHO prequalified are listed in the WHO list of prequalified in vitro diagnostic products, and are added as within-class products by WHO/GTB (18).
Table 2.3. Definition of the class of LC-aNAATs
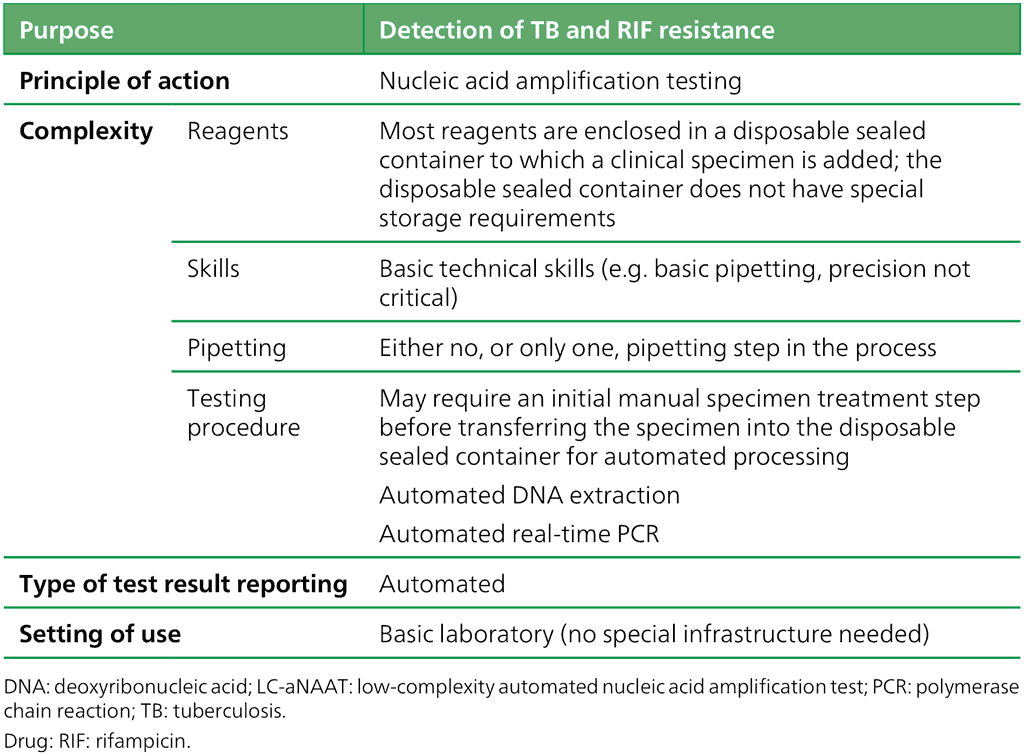
WHO recommends the use of the LC-aNAAT class for detecting pulmonary TB and extrapulmonary TB in adults, children (defined as those aged <10 years), and people (of all ages) living with HIV (Box 2.1). For adult pulmonary TB, the summary sensitivity was 90.4% (95% confidence interval [CI]: 88.0–92.4) and the summary specificity was 94.9% (95% CI: 93.0–96.3). The two tests that meet the criteria for inclusion in the class – Xpert MTB/RIF Ultra (Xpert Ultra) and Truenat MTB Plus with MTB-RIF Dx – also detect resistance to RIF. For Xpert Ultra, this is achieved by integrating the detection of MTBC and mutations in the RIF-resistance determining region (RRDR) in one cartridge. For the Truenat test, reflex testing with the MTB-RIF Dx assay should follow a positive MTB Plus result. The class summary sensitivity for the detection of RIF resistance is 95.1% (95% CI: 83.1–98.7) and the summary specificity is 98.1% (95% CI: 97.0–98.7).
When using LC-aNAATs to diagnose TB among children, concurrent testing of one respiratory specimen (i.e. sputum, gastric aspirate or nasopharyngeal aspirate) and one stool sample is recommended. If a child is HIV-positive, the concurrent testing strategy should also include LF-LAM testing on a urine sample (see Section 3.4). Concurrent testing of a respiratory sample (using LC-aNAAT) and urine (using LF-LAM) is also recommended for adults and adolescents living with HIV (see Section 3.4).
LC-aNAATs are also recommended for the detection of TB meningitis using cerebrospinal fluid (CSF), and extrapulmonary TB using lymph node aspirates, pleural tissue, pleural fluid, synovial aspirates, peritoneal fluid or pericardial fluid. It is important to stress that the bacillary load of extrapulmonary samples is often low; therefore, samples with sufficient volumes for testing should be collected and concentrated, where possible, to reach a detectable level of bacterial DNA.
When implementing these tests, users should be aware that the instruments required to run the tests have different requirements for laboratory infrastructure. For example, the GeneXpert instrument requires a dust-free environment, a stable electricity supply, and a maximum temperature of 30 °C, whereas the Trueprep and Truelab instruments can be battery operated and are less sensitive to the surrounding temperature (see Table 2.2). Some LC-aNAAT instrumentation may also be used to test for other diseases (e.g. severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], HIV, and hepatitis B and C), which can facilitate one-stop-shop services for patients and cost-sharing measures between disease programmes.
Staff running the tests need to have a basic understanding of the principles of molecular testing and know how to operate a computer. Low-complexity tests may have different pipetting requirements; for example, Xpert MTB/RIF Ultra does not require precision pipetting, whereas Molbio Truenat MTB Plus requires precision pipetting of a small volume of liquid. Maintenance and annual calibration are required; thus, manufacturers or countries often train “super users” to oversee or perform a basic service and annual calibration. Service contracts with manufacturers are also available, some of which bundle instrument service and maintenance costs into a transparent per-test unit cost.

2.2.2 Moderate-complexity automated NAATs
The MC-aNAATs are defined in Table 2.4.
Table 2.4. Definition of the class of MC-aNAATs
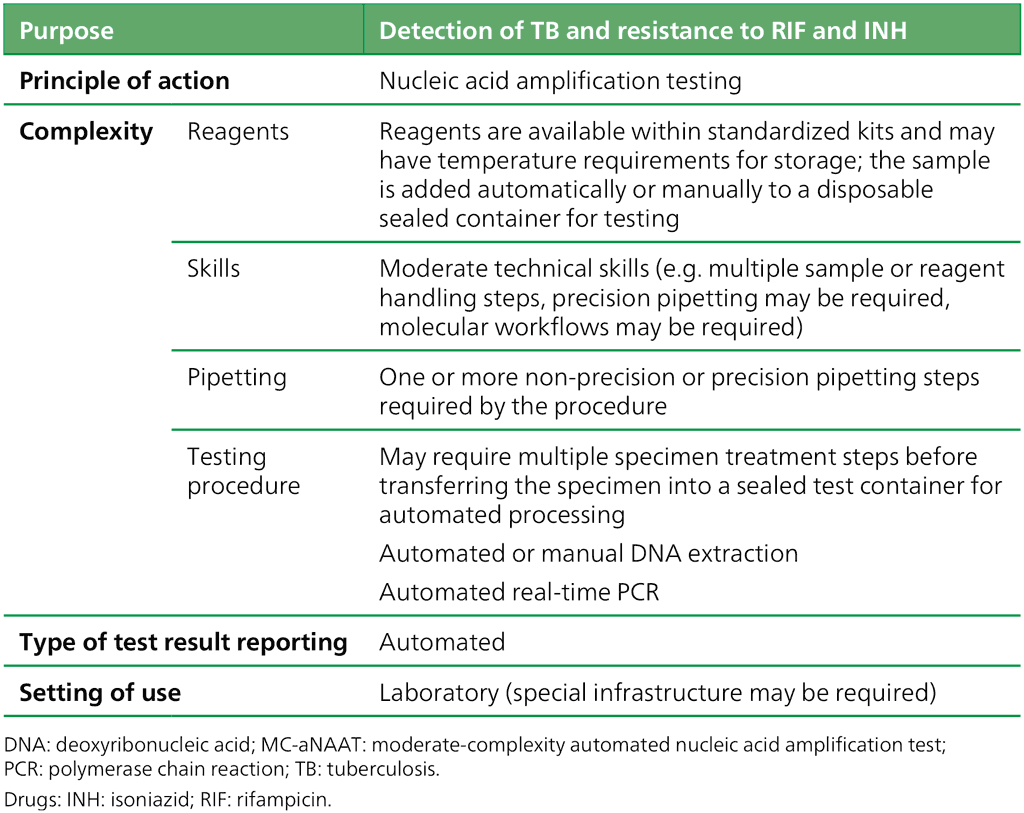
The class of MC-aNAATs includes rapid and accurate tests for the detection of pulmonary TB from respiratory samples (Box 2.2). Overall pooled sensitivity for TB detection was 93.0% (95% CI: 90.9–94.7%) and specificity was 97.7% (95% CI: 95.6–98.8%) (Table 3.1, Table 3.2 and Table 3.3 in Section 3). MC-aNAATs can simultaneously detect resistance to both RIF and INH and are less complex to perform than phenotypic DST and LPAs. After the sample preparation step, the tests are largely automated. Overall pooled sensitivity for detection of RIF resistance was 96.7% (95% CI: 93.1–98.4%) and specificity was 98.9% (95% CI: 97.5–99.5%). Fig. 2.1 illustrates the procedures for each test. Overall pooled sensitivity for detection of INH resistance was 86.4% (95% CI: 82.8–89.3%) and specificity was 99.2% (95% CI: 98.1–99.7%).
These assays offer high throughput testing and are suitable for high workload settings, so could potentially be used in areas with a high population density or TB prevalence. However, the class is primarily for laboratory settings and will require a reliable and rapid system for sample referral and result reporting. MC-aNAATs may already be used programmatically for other diseases (e.g. SARS-CoV-2, HIV, and hepatitis B and C), which could potentially facilitate implementation of TB testing on shared platforms. Information sheets summarizing the individual technologies in this class are available in Web Annex A. A detailed comparison of the different products is also available (15).
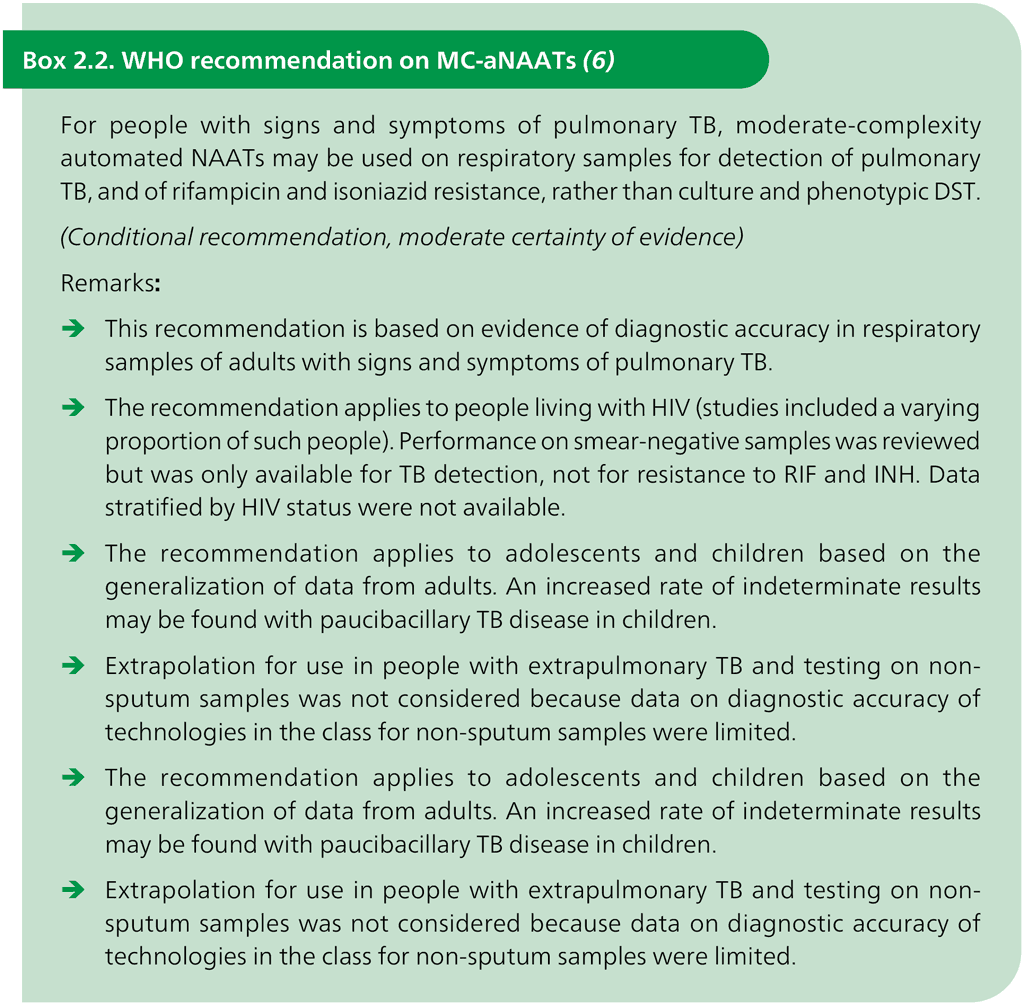
Fig. 2.1. Summary of testing procedures for the Bruker Hain FluoroType MTB and MTBDR
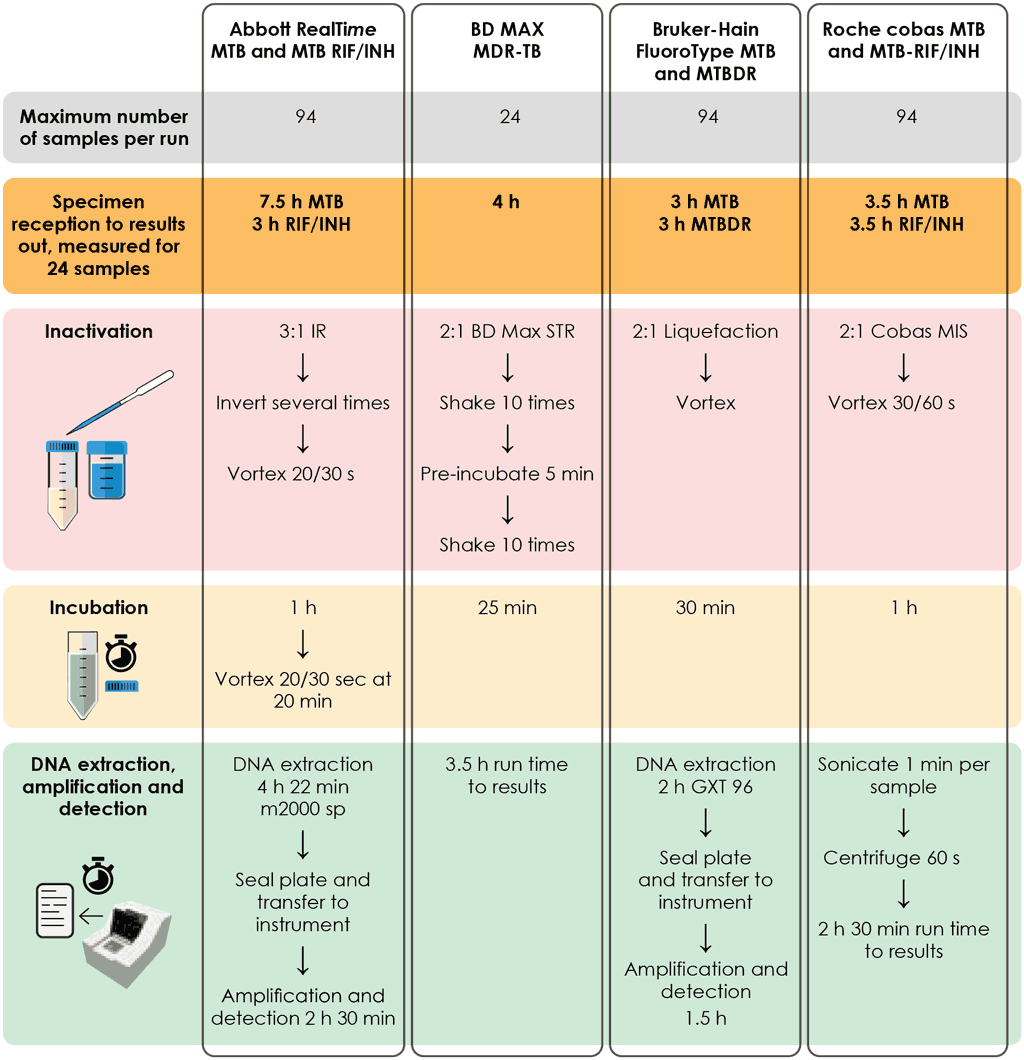
DNA: deoxyribonucleic acid; MC-aNAAT: moderate-complexity automated nucleic acid amplification test.
Source: Sengstake & Rigouts (2020) (19).

 Feedback
Feedback