Book traversal links for WHO TB KNOWLEDGE SHARING PLATFORM
It is estimated that about a quarter of the world’s population is infected with Mycobacterium tuberculosis – the bacterium that causes tuberculosis (TB) disease. Testing for TB infection can identify individuals who would benefit the most from TB preventive treatment (TPT). However, despite the availability of preventive measures and disease treatment, TB remains a leading cause of death due to a single infectious agent. TB has probably replaced coronavirus disease (COVID-19) as the leading cause of death worldwide for the first time since the start of the global pandemic (1).
In recognition of the need to end TB globally, the United Nations (UN) held the world’s first highlevel meeting on TB in 2018. The political declaration from the meeting included commitments by Member States to achieving four new global targets (2), which were subsequently renewed at the second UN high-level meeting in 2023. The commitments included two that relied on diagnosis of TB infection and disease: providing TPT to at least 45 million people between 2024 and 2027, and reaching 90% of the estimated number of people who develop TB with quality-assured diagnosis and treatment from 2023 to 2027 (2). These commitments align with the World Health Organization’s (WHO’s) End TB Strategy, which calls for the detection of individuals living with TB infection who are at higher risk of progression to active TB so that they can receive TPT, as well as the early diagnosis of TB and drug-resistant TB (DR-TB) through universal drug susceptibility testing (DST). These global commitments and plans highlight the critical role of TB testing for the rapid and accurate detection of TB infection, disease and drug resistance (3).
To support countries in their efforts to strengthen detection of TB infection, disease and drug resistance, the WHO Global TB Programme issues evidence-based policy guidance on TB testing strategies and technologies; this guidance is routinely updated. Since the most recent consolidated guidelines on TB diagnosis were issued in 2024:
- new evidence has become available on the use of WHO-recommended rapid diagnostic tests (WRDs) for the initial detection of TB and resistance to rifampicin among populations that are at increased risk of TB-related morbidity and mortality (e.g. people living with HIV andchildren);
- a systematic assessment of evidence on molecular WRDs (mWRDs) previously recommended as individual products was completed to determine the placement of mWRDs within existing or new classes of TB diagnostic technologies; and
- a call from countries was received to combine the policy guidance on TB infection, disease and drug-resistance testing into these consolidated guidelines on TB diagnosis, to streamline implementation of national testing programmes.
In response, this document is being issued as the fourth edition of the consolidated guidelines on TB diagnosis. When compared with the third edition (issued in 2024), this guideline is the first to combine the WHO policy guidance on diagnosis of TB infection, disease and drug resistance into a single reference document; also, it establishes two new classes of TB diagnostic technologies (for the initial detection of TB and resistance to rifampicin), and outlines new recommendations on concurrent testing of respiratory and non-respiratory samples among adults and adolescents with HIV, children with HIV, and children without HIV or with unknown HIV status. The main changes from the previous WHO guidelines are summarized in Box A.
The set of 21 new and existing recommendations for diagnosis of TB infection, disease and DR-TB are presented in Table A. These recommendations supersede those presented in previous editions of the guidelines and are supported by updated operational guidance that is published as the fourth edition of the WHO operational handbook on tuberculosis. Module 3: diagnosis. The operational handbook includes further details on the individual tests that are recommended for use; the selection, introduction and implementation of tests for TB infection, diagnosis and drug resistance; and updated diagnostic algorithms that reflect the updates contained within these guidelines.
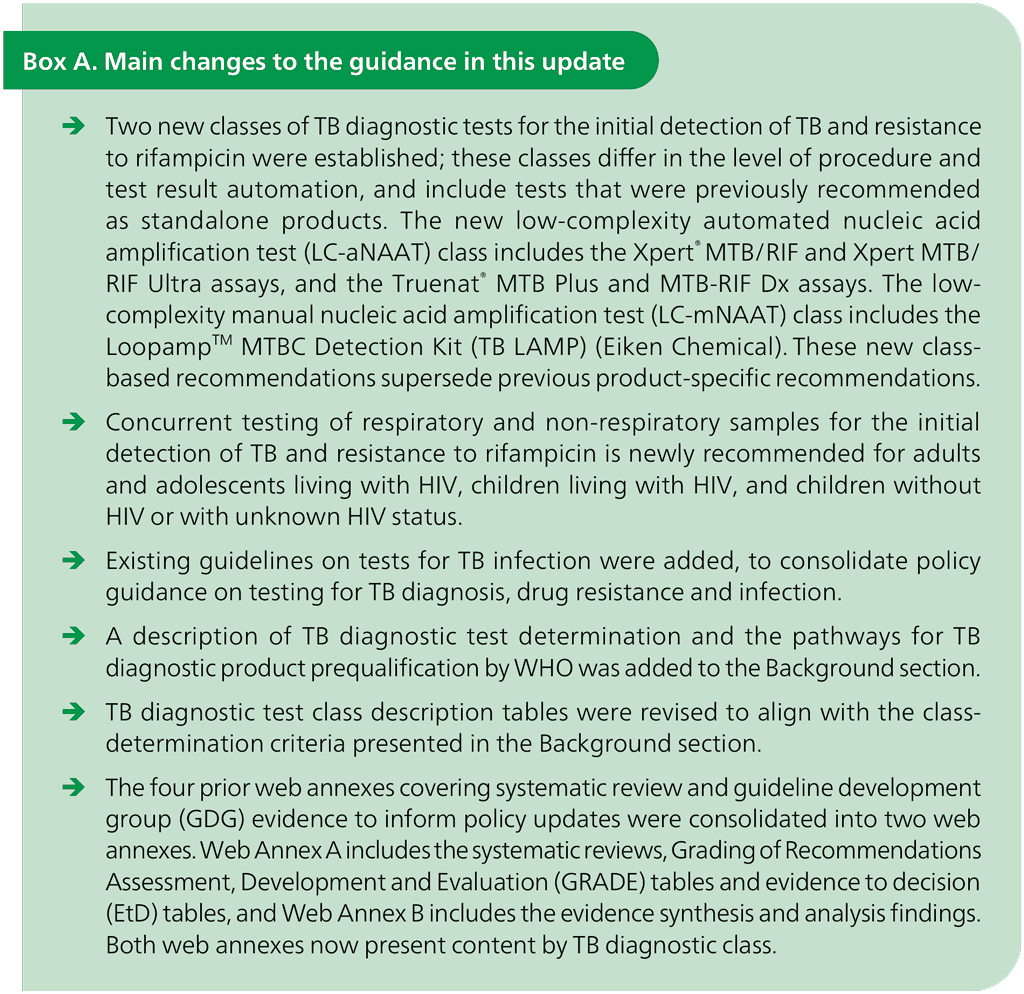
Table A. Recommendations in the WHO consolidated guidelines on tuberculosis. Module 3: diagnosis, fourth edition

DNA: deoxyribonucleic acid; DST: drug susceptibility testing; HIV: human immunodeficiency virus; LF-LAM: lateral flow urine lipoarabinomannan assay; LPA: line probe assay; MDR/RR-TB: multidrug-resistant or rifampicin-resistant TB; Mtb: Mycobacterium tuberculosis; MTBC: Mycobacterium tuberculosis complex; NAAT: nucleic acid amplification test; NGS: next-generation sequencing; SL-LPA: second-line line probe assay; SLID: second-line injectable drug; TB: tuberculosis; WHO: World Health Organization.
1 Having a positive result of a test, examination or other procedure used to distinguish people with a high likelihood of having TB disease from people who are highly unlikely to have TB. At present, the following tests are WHO-recommended as the screening tests: chest radiography (chest X-ray; CXR) with or without computer-aided detection (CAD), C-reactive protein (CRP) in people living with HIV, and molecular WHO-recommended rapid diagnostic test for TB (mWRD) (https://www.who.int/publications/i/item/9789240022676).
2 A bacteriologically confirmed TB case is one from whom a biological specimen is positive by smear microscopy, culture or WRD (such as Xpert MTB/RIF). All such cases should be notified, regardless ofwhether TB treatment has started (https://www.who.int/publications/i/item/9789241505345).

 Feedback
Feedback