Book traversal links for 1266
As highlighted above, all technologies with a WHO/GTB recommendation are expected to undergo prequalification assessment, as available. Successful assessment will be required to maintain a WHO/GTB recommendation. The current set of TB diagnostic testing classes and included products are listed in Table 1.1.1, and the two new classes are discussed below.
Table 1.1.1. Classes and products of TB tests for detection of TB, drug-resistant TB and TB infection included in the current guidelines
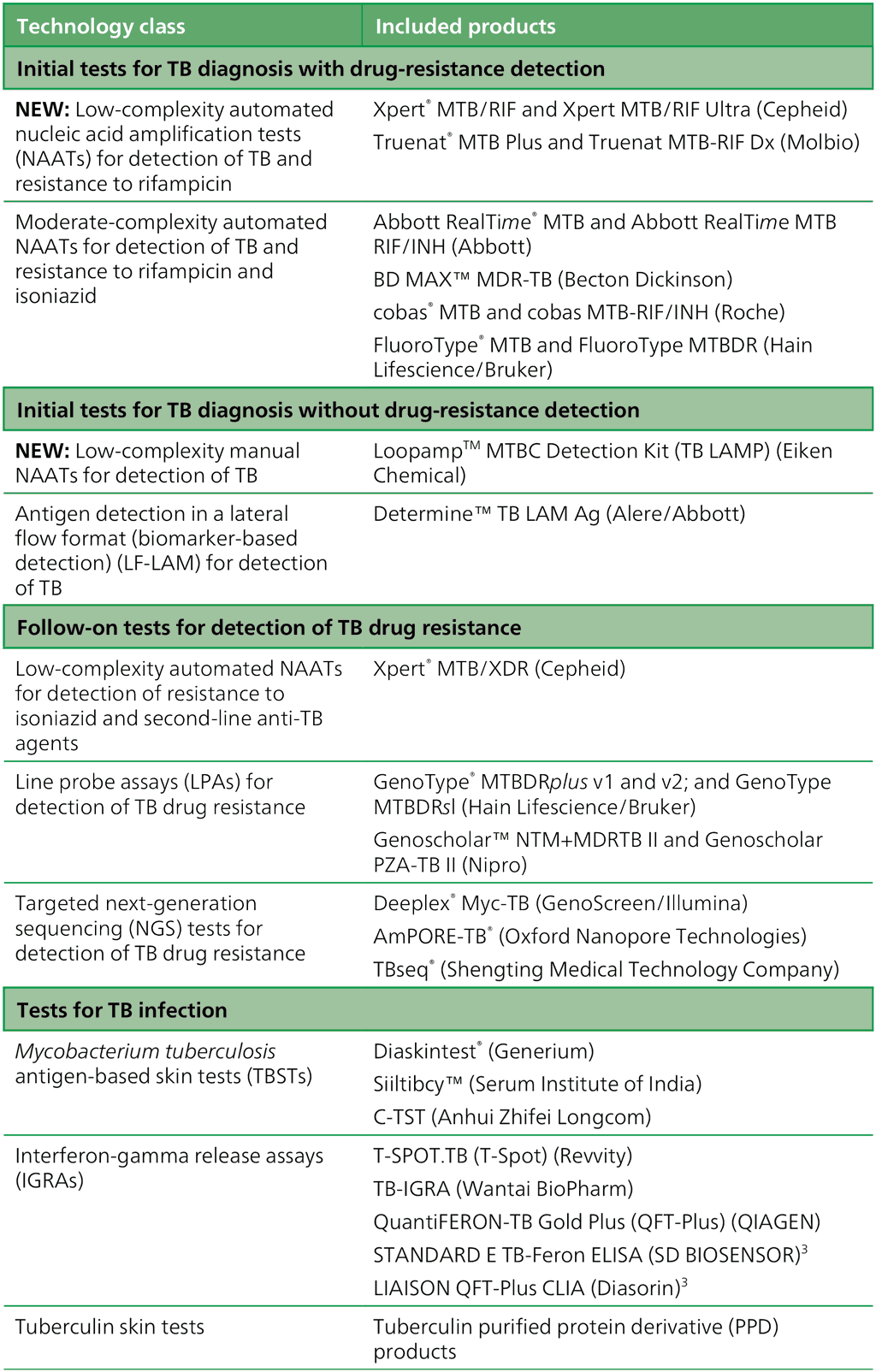
NAAT: nucleic acid amplification test; TB: tuberculosis.
1.3.1 Initial tests for TB diagnosis with drug resistance detection
Low-complexity automated nucleic acid amplification tests (NAATs) for detection of TB and resistance to rifampicin
The low-complexity automated nucleic acid amplification tests (LC-aNAATs) include tools such as Xpert® MTB/RIF Ultra (Cepheid) and Truenat® MTB Plus with MTB-RIF Dx (Molbio). These tests provide largely automated solutions suitable for decentralized laboratories, and are currently the most widely used tests for the initial detection of TB and resistance to rifampicin. The testing instruments use software and hardware (computers) to report results, and they require well-established laboratory networks and trained personnel.
Moderate-complexity automated NAATs for detection of TB and resistance to rifampicin and isoniazid
The moderate-complexity automated NAATs (MC-aNAATs) are faster and less complex to perform than phenotypic culture-based DST and line probe assays (LPAs), and are largely automated after the sample preparation step. They may be used as an initial test for simultaneous detection of TB and resistance to rifampicin and isoniazid. This type of NAAT offers the potential for rapid provision of accurate results and for testing efficiency where high volumes of tests are required daily. Hence, they are suited to areas with a high population density and rapid sample referral systems.
1.3.2 Initial tests for TB diagnosis without drug resistance detection
Low-complexity manual NAATs
The low-complexity manual NAAT (LC-mNAAT), loop-mediated isothermal amplification (LAMP), is based on DNA amplification at a single temperature range; this contrasts with the polymerase chain reaction (PCR), which requires a thermocycler. Detection of amplified product is done visually, using an ultraviolet (UV) lamp, directly in the reaction tubes. The method requires only basic equipment and can be implemented at the lowest levels of the laboratory network. However, detection of mutations in resistance-associated genes is not available with the currently recommended technology.
Antigen detection in a lateral flow format (biomarker-based detection)
The currently available lateral flow urine lipoarabinomannan assay (LF-LAM) has suboptimal sensitivity and specificity; thus, it is not suitable as a diagnostic test for TB in all populations. However, in contrast to traditional diagnostic methods, the urine LF-LAM assay demonstrates improved sensitivity for the diagnosis of TB among individuals coinfected with HIV.
1.3.3 Follow-on tests for detection of TB drug resistance
Low-complexity automated NAATs for the detection of resistance to isoniazid and second-line anti-TB agents
The LC-aNAATs are recommended for use as a reflex test in specimens determined to be positive for Mtb complex (MTBC); these tests offer rapid DST in intermediate and peripheral laboratories. The first product in this class simultaneously detects resistance to isoniazid, fluoroquinolones, ethionamide and amikacin. Results are available in under 90 minutes; this is faster than with the current standard of care, which includes LPAs and culture-based phenotypic DST.
Line probe assays
LPAs are a family of DNA strip-based tests that can detect the MTBC DNA and determine its drug-resistance profile. The tests do this through the pattern of binding of amplicons (DNA amplification products) to probes that target specific parts of the MTBC genome; that is, common resistance-associated mutations to anti-TB drugs or the corresponding wild-type DNA sequence (5). LPAs are technically more complex to perform than the Xpert MTB/RIF assay; however, they can detect resistance to a broader range of first-line and second-line agents, and they provide mutation-specific data for common variants. Testing platforms have been designed for a reference laboratory setting and are most applicable to high TB burden countries. Results can be obtained in 5 hours (5).
Targeted next-generation sequencing tests
Tests based on targeted next-generation sequencing (NGS) are used for follow-on detection of resistance to a broad range of anti-TB drugs after the initial detection of TB or of rifampicin resistance. This class of tests is based on technology that combines amplification of selected genes with NGS to detect resistance to many drugs with a single test. Because targeted NGS can interrogate entire genes to identify specific mutations associated with resistance, the accuracy may be better than that of existing WHO-recommended rapid diagnostic tests (WRDs). In addition, new tests based on targeted NGS can detect resistance to new and repurposed drugs that are not currently included in any other molecular assays. Hence, this class of tests offers great potential to provide comprehensive resistance detection matched to modern treatment regimens.
1.3.4 Tests for TB infection
Mtb antigen-based skin tests
Mtb antigen-based skin tests (TBSTs) are used for the indirect detection of TB infection. TBSTs rely on intradermal injection of Mtb-specific antigens; the antigens elicit a localized skin reaction in infected individuals that is detected by measurement of a local induration 48–72 hours after administration. Although these tests continue to rely on patient injection and return visits for result interpretation, they are more specific than the WHO-recommended tuberculin skin tests (TSTs).
Interferon-gamma release assays
Interferon-gamma release assays (IGRAs) are in vitro blood-based tests that are used to indirectly test for TB infection. They do this by measuring either the amount of interferon-gamma that is released by lymphocytes in whole blood after exposure to Mtb-specific antigens or the number of T-lymphocytes within the whole blood that produce interferon-gamma. IGRA testing requires days to perform owing to the blood incubation steps and it can be challenging to perform among patients for whom phlebotomy can be difficult (e.g. children); however, this is the only type of test for TB infection in which the results are not affected by prior bacille Calmette–Guérin (BCG) vaccination for TB. Hence, IGRAs are a promising alternative for detection of TB infection in settings with high rates of BCG vaccination.
Tuberculin skin tests
TSTs were the first class of tests to be recommended for detection of TB infection; they rely on intradermal injection of a mix of antigens to Mtb, non-tuberculous mycobacteria and the BCG vaccine formulation, followed by detection of a localized skin induration-based response after 48–72 hours. As with the TBSTs, these tests can facilitate TB infection testing in children and other patients for whom phlebotomy is challenging, but they may also produce false positive results in people infected with mycobacteria other than TB and in those who are BCG vaccinated.

3 For WHO statement and evidence assessment on new IGRAs see WHO operational handbook on tuberculosis. Module 3: diagnosis.

 Feedback
Feedback